October 5, 2007 — IntraOp Medical Corp. a provider of technology solutions for the treatment and eradication of cancer, recently announced the introduction of the Optimal Boost approach to breast cancer treatment administered by its flagship product, Mobetron.
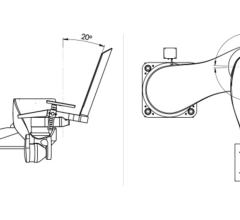
Mobetron is a fully-portable, self-shielding electron-beam linear accelerator that delivers radiation therapy in the operating room at the time of cancer surgery. It administers intraoperative electron-beam radiation therapy (IOERT) directly to the tumor bed once a cancerous growth has been excised during surgery. The Optimal Boost method, or as it is commonly referred to in breast cancer treatment, the Bio-Boost method, consists of applying a high dosage of radiation to the tumor bed at the time of lumpectomy followed by traditional post-operative radiation therapy.
Because it does not have to pass through healthy skin, organs and tissue, IOERT can reportedly be delivered at higher and better cancer-killing doses. Guided by the radiation oncologist's clear view of the site as well as intraoperative ultrasound, IOERT also allows the electron beam to be more accurately targeted In addition, IOERT eliminates the traditional delay of external radiation therapy, which must be scheduled only after the surgery patient has healed. This can give cancer cells a chance to reconstitute and spread.
For more information: www.intraopmedical.com


 June 14, 2024
June 14, 2024