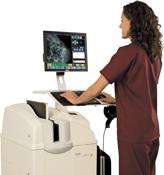
January 15, 2009 - Carestream Health installed its two thousandth high-resolution CR system for mammography at the Rechts der Isar Clinic in Munich, Germany, for breast cancer diagnosis.
The KODAK DIRECTVIEW CR Mammography feature enables mammography images to be captured digitally while utilizing a healthcare provider’s existing mammography x-ray unit and workflow processes and is intended for use in the same clinical and screening applications as traditional screen-film based mammography systems.
The optional mammography feature, available when purchasing a new Elite, Classic or CR 975 System from Carestream Health, or as a retrofit to installed Elite, Classic or CR 850/950/975 systems, allows the CR system to be used for both general radiography and mammography exams. In addition, the optional KODAK DIRECTVIEW CR Mammography Total Quality Tool is available to streamline objective mammography image testing and QC measurements.
The Munich University Rechts der Isar Clinic, with its specialized team of physicians dedicated to mammography, installed a new multi-plate KODAK DIRECTVIEW CR 975 System with Mammography Feature in November. The clinic is part of the Institute for X-ray Diagnosis of the University of Munich, which performs more than 100,000 medical procedures each year and features a fully digital environment.
Carestream Health has applied to the U.S. Food and Drug Administration for approval to market its CR system for mammography in the United States.
For more information: www.carestreamhealth.com


 February 18, 2026
February 18, 2026 









