June 2, 2008 - The ethics committee of the Centro Cardiologico Monzino (CCM-University of Milan), Milan, Italy, has approved the planned randomized clinical trial to evaluate the use of RenalGuard in the prevention of contrast-induced nephropathy (CIN) in high-risk patients undergoing catheterization procedures at its institution.
The investigators for the trial are Antonio L. Bartorelli, M.D., Director, Interventional cardiology, CCM, and professor of cardiology, University of Milan, and Giancarlo Marenzi, M.D., chief, Intensive Cardiac Care Unit, CCM, who are two of the world's leading experts in the prevention of CIN.
The trial is designed as a prospective, open, randomized trial to provide an assessment of the potential benefits of induced diuresis with matched hydration therapy, compared to standard overnight hydration, in the prevention of CIN in patients undergoing cardiac catheterization procedures and percutaneous coronary interventions with baseline impairment in renal function. The CIN-prevention therapy of induced diuresis and matched hydration therapy will be provided using RenalGuard.
"Contrast-Induced Nephropathy is a risky complication resulting from the use of contrast media for coronary and peripheral vascular diagnostic and interventional procedures in at-risk patients," stated Dr. Bartorelli. "Previous studies at our institution have demonstrated the benefit of hemofiltration in preventing CIN in patients with chronic renal failure. Our objective with this study is to assess the potential benefits of induced diuresis with matched hydration therapy compared to standard overnight hydration for the prevention of CIN."
Dr. Marenzi added, "CIN is a major life-threatening issue for at-risk patients undergoing imaging procedures since it can result in longer hospital stays and higher mortality rates. A cost-effective, easy-to-use preventive measure could save lives and reduce costs."
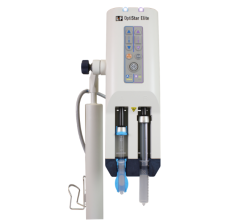
RenalGuard is based on existing study data that suggests that initiating and maintaining high urine output during imaging procedures allows the body to rapidly eliminate toxins in contrast media, reducing their harmful effect. RenalGuard is a fully-automated, real-time matched fluid replacement device intended for interventional cardiology and radiology patients undergoing imaging procedures using contrast media.
In the U.S., PLC recently completed a pilot safety study of RenalGuard. Based upon the positive safety data collected in the pilot study and discussions with FDA, PLC stopped enrolling new patients in the pilot study and has received FDA conditional approval to commence a U.S. pivotal trial to study the effectiveness of its RenalGuard in the prevention of CIN.
For more information: www.plcmed.com


 October 09, 2025
October 09, 2025