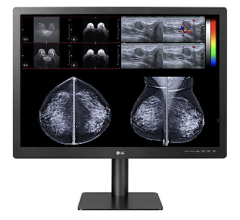
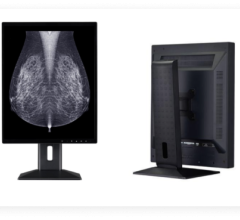
The FDA gave 510(k) clearance to the Totoku ME551i2 display for use in all digital mammography applications.
The monochrome panel, a 21.3-inch, 5-megapixel, DICOM-compliant diagnostic display, has an 11.9-bit grayscale palette and 750 cd/m2 brightness. Features also include the potential for accurate luminance uniformity across the screen, which is designed to reduce luminance variance specification by more than 50 percent when compared to uncorrected panels.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now


 July 30, 2025
July 30, 2025