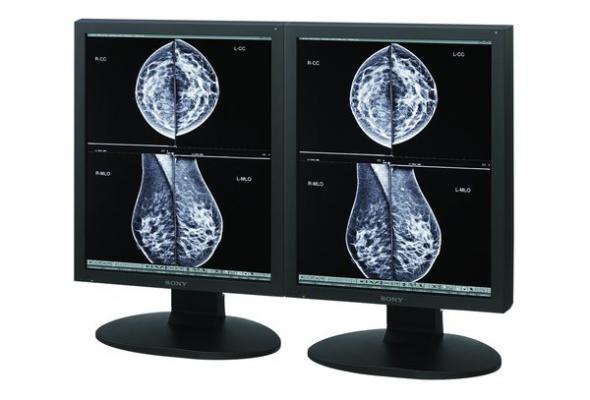
August 12, 2014 — Sony Electronics’ Medical Systems Division is showcasing its full line of approved radiology monitors, including the LMD-DM50, LMD-DM30C and LMD-DM30 models, at the 2014 annual meeting of the Association for Medical Imaging Management (AHRA) in Washington, D.C. The monitors are cleared by the U.S. Food and Drug Administration (FDA) for use in diagnosis of full-field digital mammography exams (FFDM), providing detailed viewing of microcalcifications.
Providing radiologists with independent sub-pixel drive technology that provides three times the monitor’s native resolution, the grayscale Sony displays shown at AHRA are ideal for any hospital or imaging center. The technology enables the LMD-DM30 with independent sub-pixel drive software installed on the modality to output 9MP resolution, while the 5MP LMD-DM50 is able to achieve a 15MP resolution. In addition to being used for FFDM, the independent sub-pixel drive creates superb image quality for viewing high-resolution diagnostic studies including CR (computed radiography) and DR (digital radiography) images, CT (computed tomography) and MRI (magnetic resonance imaging).
The LMD-DM30C is a 3MP color display with high resolution designed for accurate display of color and monochrome images such as PET/CT (positron emission tomography/CT), nuclear medicine, cardiology and ultrasound. With full DICOM GSDF Part 14 conformance, automatic calibration, and remote monitoring and maintenance capabilities, the LMD-DM30C is suited for medical imaging and radiology department PACS (picture archiving and communications systems).
“Radiology administrators need high-quality, reliable and cost-effective equipment, and Sony’s monitors deliver in all of these areas,” said Lida Trupp, marketing manager, Sony Electronics’ Medical Systems Division. “Our expertise with innovative display technologies enables us to bring the optimal systems to the radiology environment, so that hospitals and imaging centers can enhance and streamline their imaging workflow.”
For more information: www.sony.com/medical

 March 12, 2024
March 12, 2024