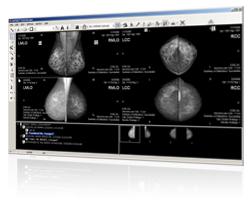
March 13, 2009 – RamSoft will be featuring its PowerServer PACS plus Digital Mammography solution at NCBC 2009 next week.
The PowerServer allows imaging professionals to read digital mammography anywhere with its reportedly fast and efficient Web-based PowerReader diagnostic workstation. Mammography specific image management and reporting tools that are typically only found on a dedicated workstation are available to everyone with PowerServer – no matter the location. Plus, RamSoft’s integration with MagView mammography reporting system aims to make workflows seamless and efficient.
RamSoft PowerServer PACS-ranked No. 2 in the PACS (Ambulatory) market segment in the 2008 Top 20 Best in KLAS Awards: Software & Professional Services report. RamSoft’s Digital Mammography solution is FDA approved for mammography, and features iCAD and Hologic CAD support, customizable viewing protocols, intelligent routing and pre-fetching. It is compatible with all modalities, scalable across multiple facilities and is IHE, HL7 and DICOM compliant.
For more information: www.ramsoft.com


 November 29, 2025
November 29, 2025 









