October 30, 2018 — Combinations of radiation and chemotherapy drugs have been shown to cure human papilloma virus (HPV)-related head and neck cancer with a high success rate. A new phase III trial has now determined that cisplatin chemotherapy, combined with radiation therapy, produces the best results and should be considered the standard of care. Findings were presented at the American Society for Radiation Oncology (ASTRO) 2018 meeting in October.
“We’ve now established that high-dose cisplatin chemotherapy — in combination with radiation — is the standard of care for human HPV-related oral cancers,” said Andy Trotti, M.D., a radiation oncologist at the Moffitt Cancer Center in Tampa and co-lead investigator of NRG Oncology/RTOG 1016. “Prior to this study, there were no definitive, ‘state of the art’ trials in this specific cancer population.”
The number of oral cancer cases associated with HPV has risen over the past several decades, even as rates for other head and neck cancers generally have declined. From 1988 to 2004, HPV-associated oropharyngeal squamous cell cancer (OPSCC) incidence rose more than 200 percent, while HPV-negative disease rates dropped by half.
The profile of the typical OPSCC patient has changed, as well. HPV-associated OPSCC is five times more prevalent among men than women and is the most common HPV-related cancer. It is the fastest-rising cancer among young, white men in the United States, and, unlike HPV-negative oropharyngeal cancers, is more prevalent among non-smokers than smokers. This type of cancer affects the back of the throat, including the base of the tongue and tonsils.
Survival rates are high — the estimated risk of death is 50 percent lower among patients with HPV-associated OPSCC than among those with HPV negative disease — in part because patients tend to be younger and healthier at diagnosis. However, serious, adverse side effects from treatment often occur. Trotti and his colleagues were exploring whether the cetuximab/radiation combination would be less toxic to patients than treatment combining radiation with cisplatin, without lowering survival rates.
“We had hypothesized that survival with cetuximab might be very close (within 5 percent) to that of cisplatin, but that was not the case.” explained Trotti.
In this phase III trial, funded by the National Cancer Institute (NCI), 805 patients with locoregionally advanced HPV-related oropharynx cancer were randomly assigned (1:1) to two cycles of cisplatin chemotherapy (100 mg/m2) every three weeks plus radiation therapy, or the same radiation therapy with weekly cetuximab treatments. Ninety percent of the patients were men, with a median age of 58.
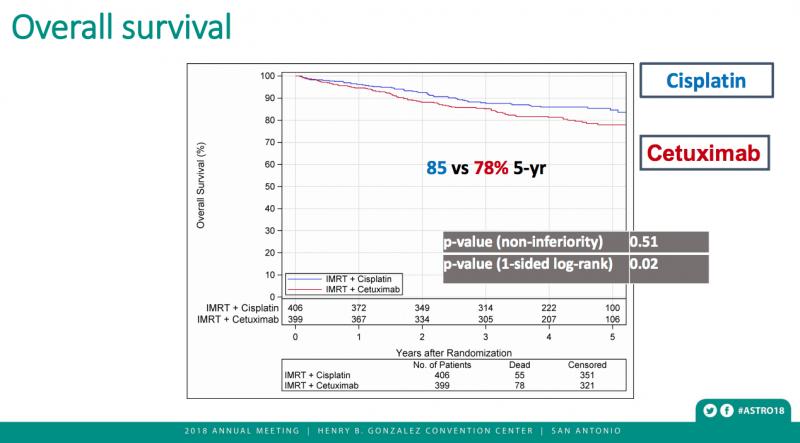
The results were released early when an interim data analysis found cetuximab with radiation was associated with inferior overall survival (hazard ratio (HR) 1.45, 95% CI 1.03, 2.05) and progression-free survival (HR 1.72, 95% CI 1.29, 2.29), compared to the cisplatin/radiation treatment combination, with five-year estimates of 78.4 percent (95% CI 73.8, 83.0) for cisplatin and 67.3 percent (95% CI 62.4, 72.2) for cetuximab.
Estimated five-year local-regional failure/distant metastases rates were also considerably lower with radiation plus cisplatin (9.9/8.6 percent) than with radiation plus cetuximab (17.3/11.7 percent). The estimated five-year survival rate was substantially better (84.6 percent) in the cisplatin group compared to the cetuximab group (77.9 percent).
Using traditional toxicity reporting methods, researchers found that patients receiving cisplatin experienced slightly more serious (grade 3-5) side effects (82 percent) overall than those treated with cetuximab (77 percent). However, said Dr. Trotti, traditional reporting of overall adverse event rates tends to obscure important differences in the magnitude of the toxicity profiles. “That is where our new metric, ‘T-score,’ does a better job of capturing the frequency of high-grade events, or toxicity burden,” he said.
Using the T-score system, all high-grade events experienced by the entire group are divided by the total number of patients. A T-score of 2.35 means the average patient had more than two high-grade events, whereas traditional reporting would only reflect one event per patient. T-score analysis showed a 40 percent higher rate of high-grade events for cisplatin, compared to a nominal five-point difference (82 vs. 77 percent) with the traditional reporting method.
The specific profile of adverse effects varied by agent, with anemia, hearing loss, nausea, vomiting, neutropenia and kidney injury more common with cisplatin, while rashes were more common among those treated with cetuximab. The rate of long-term, severe dysphagia (difficulty swallowing) was 4 percent for cisplatin, compared to 6 percent for cetuximab. Quality of life measures were collected but have not yet been reported.
Based on promising preliminary trial results published in 2006, comparing cetuximab versus radiation alone, some physicians had adopted cetuximab as a standard of care substitute for cisplatin, as a first-line option in otherwise healthy patients, said Dr. Trotti. Cetuximab may still be considered a viable treatment option for patients who cannot tolerate cisplatin, such as those with significant hearing loss or severe diabetes-related neuropathy, he added. Those conditions may be exacerbated by treatment with cisplatin.
“You want to avoid worsening patients’ hearing conditions or nerve damage,” he said. “These patients need alternative medications, such as cetuximab, which do not have overlapping toxicity with neuropathy or hearing loss.”
Last year, ASTRO released a clinical guideline for the treatment of OPSCC that noted that radiation is the most commonly used curative option for primary treatment of oropharynx tumors. The guidelines included the recommendation that patients with stage IVA-B tumors who are medically unfit for high-dose cisplatin receive concurrent cetuximab or carboplatin/5-fluorouracil.
The guideline also notes that, because of the rapid increase in incidence, the demand for radiation oncologists to treat head and neck cancer is projected to rise.
Related content: Halving Radiotherapy for HPV-Related Throat Cancer Reduces Side Effects With Similar Control Rates
Read more about the Late-breaking Radiation Therapy Clinical Trials at ASTRO 2018.
For more information: www.astro.org


 February 04, 2026
February 04, 2026 









