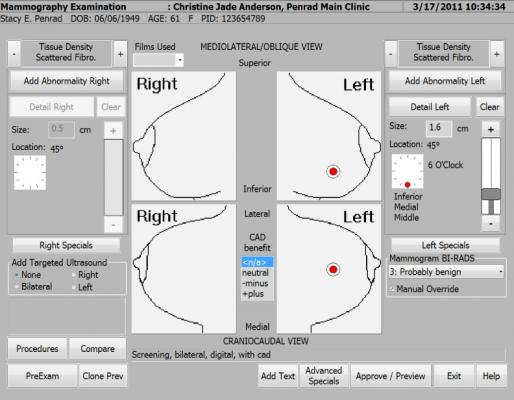
October 14, 2014 — PenRad Technologies Inc. announced its partnership with Clinithink to refine the process of clinical documentation for radiologists in real time. PenRad is embedding Clinithink’s CLiX CNLP (clinical natural language processing) platform into its MIS (mammography information system) solution to increase productivity, accuracy and efficiency of the mammography reporting workflow for its existing install base.
“Following an in-depth evaluation, we found Clinithink to be the most clinically rich, easily integrated platform available on the market today,” said Greg Gustafson, president and CEO of PenRad Technologies. “Adding Clinithink’s CLiX CNLP to PenRad’s rules engine for its MIS solution will enable our radiologists to receive instant feedback for optimum ICD-10 coding as they dictate their reports, saving valuable time and improving the accuracy of the data.”
PenRad will debut this combined technology platform to the broader radiology market in 2015. With a series of updates to its existing solutions to support both narrative and structured data and through integration with acute and ambulatory electronic medical record (EMR) vendors, this new generation of software will make the ICD-10 transition seamless, while positively impacting clinicians’ workflow and improving quality of care.
“We are excited to be working closely with a radiology powerhouse like PenRad,” said Chris Tackaberry, M.D., Clinithink CEO. “Our partnership highlights the pressing need to leverage unstructured healthcare data to address business challenges including revenue optimization, workflow management and standards of care. We are delighted that PenRad has identified the benefits that can be offered to its customers in these areas by integrating our CLiX CNLP platform.”
For more information: www.penrad.com


 February 18, 2026
February 18, 2026 









