Image courtesy of Astute Medical
January 27, 2015 — Ortho-Clinical Diagnostics Inc. announced the nationwide availability to hospitals of the Nephrocheck Test System designed to help healthcare providers identify patients at risk of developing moderate or severe acute kidney injury (AKI) within 12 hours of patient assessment.
In comparing patients with AKI to patients without AKI, hospital and the intensive care unit lengths of stay double, as do costs of care and readmission rates. Death rates at one year are higher among patients with AKI alone, compared to those patients with heart attack alone.
AKI usually lacks signs and symptoms and can potentially result in irreversible kidney damage if recognition is delayed. In clinical studies, the Nephrocheck Test identified the majority of patients that developed moderate to severe AKI within 12 hours of assessment.
The Nephrocheck Test result, called the AKIRisk Score, has the ability to distinguish patients with AKI from those without AKI. Based on results from clinical studies, patients with a positive AKIRisk Score (greater than the cutoff of 0.3) have a one in four to a one in three chance of developing moderate or severe AKI within 12 hours of assessment.
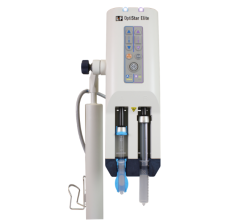
To calculate the AKIRisk Score, the Nephrocheck Test System measures the concentrations of two urinary biomarkers using the Astute140 Meter. The two novel biomarkers -- tissue inhibitor of metalloproteinase 2 and insulin-like growth factor binding protein 7, are thought to be involved in G1 cell cycle arrest in the earliest phases of injury.
In July 2014, Ortho-Clinical Diagnostics entered into collaboration with Astute Medical Inc., the developer of the Nephrocheck Test System, to become the exclusive sales agent for Astute Medical’s Nephrocheck Test and the Astute140 Meter in the United States and in certain countries of the European Union.
For more information: www.orthoclinical.com/en-us/Pages/Home.aspx, www.astutemedical.com


 October 09, 2025
October 09, 2025