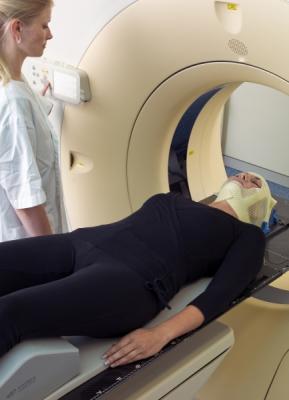
January 24, 2014 — Orfit Industries America, North American subsidiary of Orfit Industries, received U.S. Food and Drug Administration (FDA) clearance to market its Nanor hybrid thermoplastic technology for use in immobilization masks for
radiation therapy. The company estimates immobilization products using the advanced technology will become available for sale in the United States during the first half of 2014.
Orfit’s nano-technology will be used in Nanor masks, creating a mask that is 33 to 50 percent thinner than conventional masks. They are expected to exhibit little shrinkage and are light in an effort to improve patient comfort. The masks conform to a patient’s head to provide stability during radiation therapy. The masks reduce resource use in production to minimize waste and environmental impact.