Results of an independent clinical study conducted by U.S. Army combat support surgeons in Baghdad show that a new medical device used to close a type of surgical wound more quickly is saving lives and limbs of soldiers and civilians.
A team of battle zone surgeons led by Maj. Niten Singh, M.D. working in the 28th Combat Support Hospital in Baghdad during Operation Iraqi Freedom, used Canica Inc.’s ABRA Surgical Skin Closure System to successfully implement a new surgical regimen called dynamic wound closure (DWC). Described in a paper published in “The American Surgeon,” the procedure applies gentle but continuing tension uniformly across a wound for a period of a few days to gradually but completely close it. Skin grafting is eliminated.
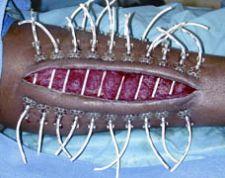
A surgeon sometimes must make a long incision to perform a fasciotomy – usually on a badly injured arm or leg – to relieve dangerous swelling and pressure due to fluid buildup. Fasciotomies must often be performed quickly following trauma and can mean the difference between loss of life or limb, but result in long, gaping wounds which can be difficult to close. More than 16,000 are performed in U.S. hospitals annually.
“Because of the swelling that occurs with the muscles, these wounds cannot typically be closed with sutures,” explained Dr. Singh, who is based at Madigan Army Medical Center, Tacoma, WA. “Traditionally, surgeons have left these wounds open and covered them with skin grafts. This results in an additional wound from harvesting the graft; potential failure of the skin graft and unsightly scars.”
“Using Canica’s system, we were able to close fasciotomies in an average 2.6 days - several times faster than other techniques,” said Dr. Singh. “Canica’s system is designed for early placement and our findings indicate early placement is key to success, allowing skin approximation to occur faster. Overall, we do believe this is a superior technique.”
“Dynamic Wound Closure: beginnings of a new standard of care,” is another recent study supports Dr. Singh’s belief that dynamic wound closure as early as possible saves more lives and limbs. The research, published in “The Journal of Trauma Injury, Infection, and Critical Care” concluded that performing fasciotomies in battlefield hospitals - rather than waiting to perform them hours or days later at Landstuhl Regional Medical Center (LRMC) in Germany resulted in three times fewer deaths and reduced the rate of amputations by half. Using dynamic wound closure, these early fasciotomies can now be rapidly and reliably closed, and secondary surgical procedures can be avoided.
Faster healing minimizes complications, patients leave the hospital sooner and civilians require less home care, the manufacturer said.
The wound closure device can be used on many types of wounds, not just fasciotomies. In addition to closing other extremity wounds, Canica makes dynamic wound closure systems for a wide range of wounds, from skin lacerations to large abdominal eviscerations.
May 2008

