January 17, 2008 - IntraOp Medical Corp. announced that the Ohio State University Comprehensive Cancer Center — James Cancer Hospital and Solove Research Institute (The James) — in Columbus, has successfully treated a liposarcoma patient using the Mobetron, which administers intraoperative electron-beam radiation therapy (IOERT) at the time of cancer surgery.
In the Fall of 2000, Diane Miesse, a buyer for the U.S. Military living in Columbus, OH, was diagnosed with liposarcoma, a malignant tumor that arises in fat cells found in deep, soft tissue. After several rounds of treatment and recurrences, Miesse was referred to The James to work with Dr. Michael Walter, a surgical oncologist, and Dr. John C. Grecula, a radiation oncologist. The doctors treated Diane using the Mobetron during the surgery to administer the IOERT treatment to target any remaining cancer cells in the area.
"The Mobetron allows us much more flexibility in the delivery of IOERT. In most cases, the Mobetron can be moved over the patient rather than moving the patient to the linear accelerator. This not only speeds up the delivery process but also increases the safety for the patient. Also the beam collimator (radiation applicator) is aligned to the linear accelerator utilizing a laser system so the sterile environment of the operative field is maintained," said Dr. Grecula.
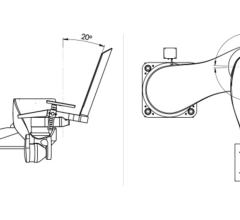
The Mobetron is an OR-ready, fully portable, self-shielding electron-beam linear accelerator designed for use in any operating room. The Mobetron is designed to help radiation and surgical oncologists pinpoint the exact area that requires radiation and immediately deliver high doses directly to the affected tissue during cancer surgery. Hospitals can wheel the Mobetron between existing operating rooms without investing in costly renovations to accommodate traditional radiation therapy devices. Key benefits of the Mobetron are said to include: better local tumor control, shorter treatment cycles, fewer side effects and increased survival rates.
For more information: www.intraopmedical.com


 June 14, 2024
June 14, 2024