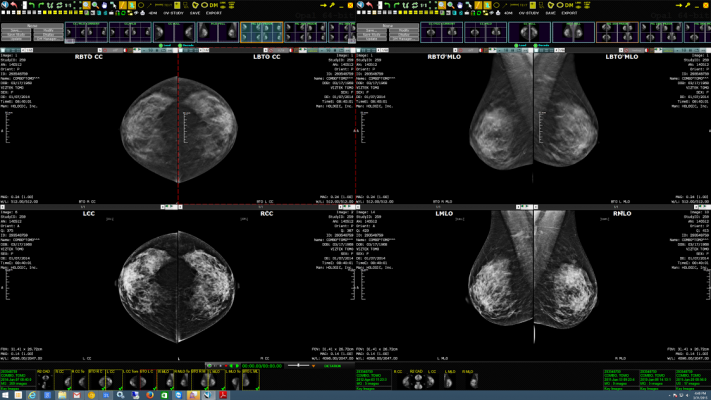
April 27, 2017 — Konica Minolta Healthcare Americas Inc. announced its participation as a sponsor of the Pre-Conference Digital Breast Tomosynthesis (DBT) Workshop at the 2017 Society of Breast Imaging (SBI)/American College of Radiology (ACR) Breast Imaging Symposium, April 6-9 in Los Angeles. The workshop provided eight hours of initial training in DBT as required by the U.S. Food and Drug Administration (FDA), and attendees had the opportunity to review and report cases on individual DBT workstations, such as Konica Minolta’s Exa Mammo.
Michael N. Linver, M.D., FACR, director of mammography at X-Ray Associates of New Mexico, P.C., and clinical professor of radiology at the University of New Mexico School of Medicine in Albuquerque, led the pre-conference workshop on April 5. The workshop included 8 Exa workstations, with a total of 40 workstations from six vendors, to help attendees learn how to read and report DBT studies and provide this advanced breast imaging service.
Konica Minolta will again support Linver by providing Exa during his Tomosynthesis Review workshop at the 22nd Annual Mammography event, August 7-11 in Santa Fe, N.M.
At the SBI/ACR workshop, 30 tomosynthesis cases were provided to each workstation vendor. With Exa’s Server-Side Rendering technology, users had fast access to the large DBT files via an internet connection to Exa, with no prefetching required. Konica Minolta’s Exa was well-received by users during the workshop.
“From the attendees, there were glowing reports about Exa. They reported it was easy to use, very ergonomic and very well-designed. I commend Konica Minolta for developing a very good product and it appears to be a winner,” said Linver.
Exa Mammo offers a diagnostic-quality zero footprint universal viewer for DICOM and non-DICOM images; Server-Side Rendering for fast access to large files, such as DBT images, with no prefetching required; and cybersecurity with no data transferred to or stored on workstations to minimize unwanted exposure to patient data. For women’s health departments and clinics seeking to consolidate viewing, Exa offers universal viewing of images from a single workstation.
For more information: www.konicaminolta.com/medicalusa


 February 17, 2026
February 17, 2026 









