November 15, 2013 — Hospital La Fe, a clinical collaborator of Nucletron, an Elekta company, is nearing the conclusion of the enrollment phase of a clinical study with the Esteya electronic
brachytherapy system for treating skin cancer. First clinical data on efficacy are expected in about two months, but physicians in the center's
radiotherapy department are already reporting that patients have tolerated the treatments well and that the system is user-friendly.
The enrollment of the first group of 20 patients ever to receive treatment with Esteya, was completed in October. The center is using a standard treatment protocol of 36.6 Gy over six fractions, 6.1 Gy per fraction delivered twice weekly.
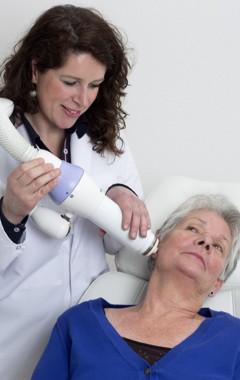
"The patients have accepted the treatment very well and side effects reported for the first treated patients have been very mild — typically just little skin reddening or itching," said José Perez-Calatayud, head of medical physics, Hospital La Fe. "The treatment delivery times have averaged less than three minutes per lesion, for a total treatment session of seven to eight minutes. This short therapy delivery is important, certainly, for patients with multiple lesions. One of the patients we treated had nine lesions."
"With five surface applicators ranging in size from 1 cm to 3 cm, we have a great opportunity to adapt to the lesion's size, thus helping us avoid exposing normal tissue," said Perez-Calatayud. "And the leakage dose — the dose outside the volume to be treated — is extremely low due to the combination of low energy and tungsten shielding of the applicators."
Planning and delivery of electronic brachytherapy at Hospital La Fe has been exceptionally straightforward.
"The graphical user interface is uncomplicated and patient setup is quite simple, contributing to a smooth, efficient workflow," said Perez-Calatayud.