March 21, 2008 - The FDA gave conditional approval to PLC Systems to begin enrollment in a U.S. pivotal trial to study the effectiveness of RenalGuard Therapy and RenalGuard System in the prevention of Contrast-Induced Nephropathy (CIN).
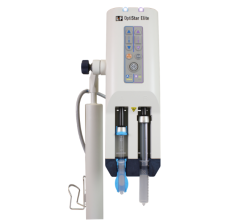
RenalGuard is based on existing pre-clinical study data that suggests that initiating and maintaining high urine output during imaging procedures allows the body to rapidly eliminate toxins in contrast media, reducing their harmful effects. RenalGuard is a fully-automated, real-time matched fluid replacement device intended for interventional cardiology and radiology patients undergoing imaging procedures using contrast media.
RenalGuard Therapy is designed to reduce the toxic effects that contrast media can have on the kidneys. The real-time measurement and matched fluid replacement design of the RenalGuard System is intended to ensure that a high urine flow is maintained before, during and after these procedures. This should allow the body to rapidly eliminate contrast, reducing its toxic effects. The RenalGuard System, with its matched fluid replacement capability, is intended to minimize the risk of over- or under-hydration.
The FDA’s conditional approval to the Investigational Device Exemption (IDE) supplement will enable the company to commence its pivotal study this spring focused on incorporating FDA’s input into our final study protocol.
Contrast-Induced Nephropathy, or CIN, is a major and growing problem due to the increasing number of older patients, diabetics and patients with pre- existing renal impairment - all of whose conditions make them at risk for CIN when they require interventional procedures that use radiographic contrast media. The U.S. pivotal study, under the supervision of principal investigators Charles Davidson, M.D., professor of Medicine, Northwestern University Medical School and Richard J. Solomon, M.D., professor of Medicine, University of Vermont College of Medicine, is designed as an adaptive, randomized controlled trial at up to 30 sites in the U.S. Enrollment in the trial is expected to last through 2009 and will include a minimum of 246 patients.
Studies indicate that approximately 15-20 percent of all patients undergoing image-guided cardiology and radiology procedures are at risk of developing CIN. The estimated mortality rate for patients that acquire CIN may be as high as 35 percent.
For more information: www.plcmed.com


 October 09, 2025
October 09, 2025