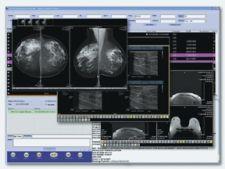
ScImage gained FDA clearance for primary diagnostic review of mammographic images using its enterprise-wide, Web-based PACS, PicomEnterprise Version 3.0, which includes default hanging protocols for standard screening exams, automated hanging protocols for prior comparison and linked window/level and pan/zoom functions.
PicomEnterprise Version 3.0 combines DICOM and HL7 functionality as part of the core server architecture. This foundation delivers several new productivity-enhancing features, including voice recognition-based reporting. Core image and information communication protocols are joined with customizable business rules aiming to create an improved workflow management foundation.
This latest innovation utilizes PicomEnterprise’s core Web services architecture to establish a foundation for improving operational efficiencies within the department, across multiple facilities and for reading groups with multifacility contracts. This version features dynamic workflow allocation that enable users to assign studies to specific readers based on location, time of day, subspecialty, individual backlogs and current workload indexes. Administrators may now also track and manage daily workflow, order backlogs and report turnaround.
January 2008


 February 24, 2026
February 24, 2026 









