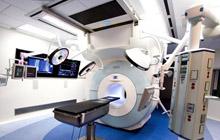
December 18, 2008 – The FDA has cleared the 3.0 Tesla IMRISneuro manufactured by IMRIS Inc. for sale in the U.S.
IMRISneuro is available with either a 1.5 Tesla or a 3T magnet. Both systems provide IMRIS' patented technology and utilize 70 cm wide bore advanced magnetic resonance imaging systems from Siemens.
The 3T IMRISneuro provides advanced imaging techniques for surgical planning such as diffusion tensor imaging (DTI), functional MRI and spectroscopy as well as vascular imaging in a platform specifically designed for use in an operating room. This additional system provides hospitals with more choice to meet the unique and growing needs of intraoperative and interventional imaging.
The 3T IMRISneuro has also been approved in Canada, with the first system delivered to Foothills Hospital in Calgary, Alberta in November 2008. Previously, Foothills Hospital led the way in intraoperative MR imaging by being the first in the world to choose the 1.5T IMRISneuro system.
For more information: www.imris.com

