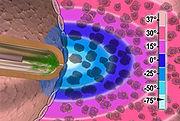
Cryoablation
February 9, 2010 - Breast imaging is on a growth track, providing an additional application for MRI and prompting the adoption of molecular imaging for breast. Similarly, minimally invasive procedures are becoming more widely adopted in interventional radiology, as techniques like cryosurgery become increasingly important for delivering therapeutic relief to patients with breast disease.
Making the most of this trend, Sanarus Technologies, LLC, recently announced it purchased the assets of Sanarus Medical, Inc., a leading developer of minimally invasive medical devices for diagnosing and treating breast disease.
Sanarus Medical is a developer of cryosurgical technologies, which uses a minimally invasive technique designed to effectively deliver therapeutic relief to breast cancer patients.
Sanarus Technologies believes this technology improves the diagnosis and treatment of breast disease, and is therefore making a significant investment in the company’s product offerings and the support.
For more information: www.sanarus.com


 February 18, 2026
February 18, 2026 









