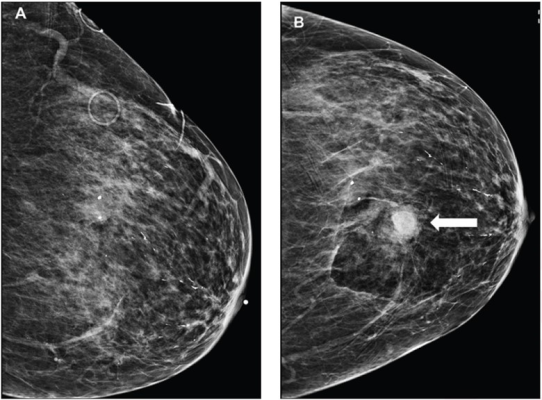
85-year-old patient who maintained daily use of 81 mg of aspirin at time of stereotactic-guided core-needle biopsy of group of breast calcifications; pathologic assessment yielded atypical ductal hyperplasia. Left craniocaudal (CC) mammographic view obtained before (A) and after (B) biopsy show hematoma on postbiopsy image (arrow), measuring 1.2 cm.
May 11, 2023 — According to an accepted manuscript published in ARRS’ own American Journal of Roentgenology (AJR), frequencies of imaging-apparent and palpable hematoma were not significantly different between patients temporarily discontinuing versus maintaining antithrombotic therapy (AT).
“The findings support safety of continuing AT during breast core-needle biopsy (CNB),” wrote lead researcher Melissa Reichman, MD, of Weill Cornell Medicine at New York-Presbyterian Hospital, adding that patients who maintain AT should be counseled regarding risk of bruise.
This AJR accepted manuscript included 5302 patients (median age, 52 years) who underwent image-guided breast or axillary CNB between January 1, 2014 and December 31, 2019. From January 1, 2014 to December 31, 2016, patients temporarily discontinued all AT for 5 days before CNB; from January 1, 2017 to December 31, 2019, the cohort maintained AT during CNB. After reviewing immediate postbiopsy mammograms for apparent hematoma, patients were then called 24-48 hours postprocedure and asked about palpable hematoma and breast bruise. Reichman et al. then reviewed medical records for clinically significant postbiopsy hematoma: hematoma requiring drainage, primary care or emergency department visit for persistent symptoms, or hospital admission. Finally, bleeding events were compared among groups.
Ultimately, imaging-apparent hematoma occurred in 3%, 6%, and 7%; palpable hematoma in 2%, 4%, and 4%; and breast bruise in 2%, 1%, and 6%, of patients without AT use, patients discontinuing AT, and patients maintaining AT, respectively, during breast CNB. The authors also reported that no patient developed clinically significant hematoma after biopsy.
Noting their findings “support the overall safety of maintaining AT during breast CNB,” Reichman and colleagues concluded that developing concomitant institutional policies and professional guidelines should be considered, too.
For more information: www.arrs.org


 March 06, 2026
March 06, 2026 









