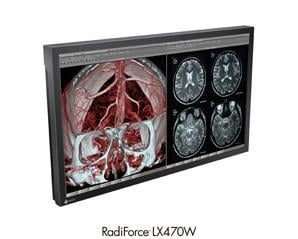
EIZO GmbH, Display Technologies expanded its portfolio of medical surgical monitors by announcing the RadiForce LX470W, a 2 megapixel widescreen monitor.
The RadiForce LX470W features a 1920 × 1080 native resolution, 47 inch screen, 1000:1 contrast ratio, 700 cd/m² brightness, and 178° viewing angles. The monitor is ideally suited for displaying DICOM X-rays or serving as a repeater monitor in surgical or endoscopic use. In addition to its versatility in the operating room, the monitor can be used for viewing images in conference and training rooms.
As with all EIZO medical monitors, the RadiForce LX470W comes precalibrated from the factory. Five integrated and calibrated look up tables (LUTs) make it possible to store gamma settings in the monitor, thus allowing the use of standard graphics cards. In all five LUTs, the medical procedure-based calibrated data is initially stored at a high resolution and, together with a wide range of possible input and output signals, allows the monitor to be used under the most varied environmental conditions.
The simultaneous display of different picture sources as Picture-in-Picture (PiP) or Picture-and-Picture (PaP) using the widescreen format provides flexible viewing of both live video or archived images anywhere reference pictures are required. The high quality RadiForce LX470W is specifically designed for 24-hour operation. The monitor’s waterproof front panel and fanless functional design provide for easy cleaning and disinfection, as well as use in a sterile hospital environment.
In the field, the monitors can be adjusted and calibrated to comply with standards such as CIE and DICOM (Digital Imaging and Communications in Medicine) using the SMfit Total Care calibration and settings tool bundled with the monitor. The RadiForce LX470W also features fully automated brightness stability thanks to EIZO’s Internal Stability System (ISS).
For customer assurance, the RadiForce LX470W complies with stringent medical, safety, and EMC emission standards. In addition, EIZO’s quality management system for medical devices is ISO 13485 certified.
Availability
Mass production of the RadiForce LX470W is scheduled for quarter one of 2010. Availability varies by country so please contact the EIZO subsidiary or distributor in your country for details.

 March 12, 2024
March 12, 2024