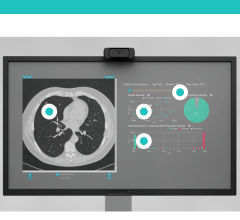
Elekta Mosaiq Evaluate is a toolset that unites plan and dose review capabilities in a single software framework, the Mosaiq Oncology Information System (OIS).
With the sheer volume of patient information growing by the minute in the healthcare industry, it is important for providers to have an information system that not only captures and maintains data, but also performs its functions effectively and meaningfully. As the electronic health record (EHR) requirements for Stage 2 meaningful use (MU) continue to bear down on clinicians, vendors — especially in the field of oncology — will need to tweak and adjust to ensure their systems fulfill the needs of providers.
Integration is Key
Though the field is dominated by a few major players, the door has been opened for enterprise vendors in the oncology information system (OIS) market. “We see the large enterprise vendors moving into the oncology space,” says Monique Rasband, oncology research director at KLAS. She adds that with more hospitals and clinics looking to bring their systems together under one roof, “oncology has become a hot topic among CIOs and clinicians who are trying to find a balance between functionality and integration.”
And integration remains key for all OIS vendors, says Rasband. With such a specialized department, it is becoming more important for systems to be able to fit in with the rest of the hospital’s network. “Oncology is a difficult animal,” she says. “This is probably why there are so few true OIS vendors in the market. It is important for these systems to be able to interface with other departments as well as the core electronic medical record in the hospital.”
Room for Improvement
In the latest Best In KLAS report for software and services, three vendors — Elekta, IntrinsiQ and Varian — were ranked as meeting the minimum KLAS Konfidence level for the oncology software market segment. Elekta’s Mosaiq system took the top spot for 2012. Another recent KLAS report, “Oncology 2012: Pulling the Curtain Back,” surveyed OIS users and healthcare providers — from physicians to information technology (IT) directors and managers to executives — on the top vendors in the market. The report found that alerts and reporting were the areas oncology providers were least happy with overall, with participants expressing that vendors’ customized reports were not as user-friendly as needed, or were particularly difficult to use.
“Reporting is another area of focus and has been a pain point in the past,” says Rasband, who authored the KLAS report on oncology. “A system could have all of the functionality in the world, but if the user is not able to easily access the data stored, it does not do much good. This information needs to be easily accessible.”
A desire for improved patient portals was also expressed in the oncology survey. As Stage 2 MU rules stress the importance of keeping patients abreast of their health information, oncology providers are weighing portals as a factor in their purchasing decisions. Patient portals provide an avenue for patients to view their health information online, as well as download and transmit their records to other providers if needed. “This needs to be accessible and simple,” says Rasband of patient portals. “When we published our last report, there was a very low adoption rate of patient portals.” The latest report indicated only 30 percent of those surveyed had adopted a patient portal, with many still needing to make a decision.
She adds that providers are in need of oncology information systems that are ahead of the game with regard to MU, as eligible participants prepare to attest for Stage 2. Varian and Epic were named as vendors that could improve for MU, while Altos and BMSi were reported as the best partners for the financial incentive program. Here, Rasband again stresses the importance of integration for OIS, since Stage 2 MU also calls for computerized physician order entry (CPOE) of medication, laboratory and radiology orders, and hospitals and clinics will want systems that streamline the various workflows together.
Latest Features and Developments
Other features are also making their way into oncology software, as well as more capabilities to integrate OIS with radiation therapy systems. In January 2013 Elekta introduced Mosaiq Evaluate, a toolset that unites plan and dose review capabilities within the company’s OIS platform. The toolset, which received 510(k) clearance from the U.S. Food and Drug Administration (FDA), streamlines planning activities inside the patient’s medical record, reducing wait times and delays as well as improving efficiency and productivity in the department. The goal of Mosaiq Evaluate is to integrate the treatment planning process with patient data and provide specific benefits for different members of the radiation oncology team, streamlining the radiotherapy workflow.
Similarly, the latest release of Varian’s Aria software, version 11, was designed to improve ease of use and interoperability with the company’s Eclipse treatment planning software. In April 2012 Varian announced a new strategic global partnership with Siemens Healthcare that includes the development of interfaces that will enable Varian’s Aria software to more easily connect with Siemens accelerators and imaging systems, giving providers more options for streamlining workflow and devising imaging and treatment solutions.
A Changing Landscape
With Stage 2 meaningful use rules bearing down and hospitals looking to integrate their information systems under one roof, there are many areas in the oncology software market for vendors to improve in. “The true blue OIS vendors are really being put to the test as they work fast and furiously to push the envelope on features and capabilities,” says Rasband.
“Traditionally, oncology was a group of physicians in a private practice who solely focused on cancer care. Now we are seeing these groups consolidated into either a larger multispecialty group or taken on by the hospital,” she adds. “The landscape is rapidly changing.”



 February 04, 2026
February 04, 2026