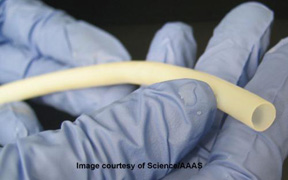
February 4, 2011 – The day when a surgeon can pull a new human vein "off the shelf" for use in life-saving vascular surgeries is now one step closer to reality. New research published in the current issue of the journal Science Translational Medicine demonstrates the efficacy of tissue-engineered vascular grafts (TEVGs) that are immediately available at the time of surgery and have decreased potential for infection, obstruction or clotting. The bioengineering method of producing veins shows promise in both large and small diameter applications, such as for coronary artery bypass graft (CABG) surgery and for vascular access in hemodialysis.
Coronary Artery Bypass Graft (CABG) Surgery
The American Heart Association Update on Heart Disease Statistics reports that in 2007, in the U.S., more than 400,000 coronary bypass procedures were performed. Patients requiring bypass surgery may not have suitable veins or arteries available and are not candidates for synthetic grafts because of the size needed for grafting.
"This new type of bioengineered vein allows them to be easily stored in hospitals so they are readily available to surgeons at the time of need," said Alan P. Kypson, M.D., associate professor of cardiothoracic surgery, Brody School of Medicine, at East Carolina University, also an author of the paper. "Currently, grafting using the patient's own veins remains the gold standard. But, harvesting a vein from the patient's leg can lead to complications, and for patients who don't have suitable veins, the bioengineered veins could serve as an important new way to provide a coronary bypass."
Kidney Hemodialysis
According to statistics published by the National Kidney Foundation, 320,000 patients are on chronic hemodialysis. Each year, 110,000 new patients develop renal failure requiring dialysis, and the number is growing by three percent per year. More than half of dialysis patients lack the healthy veins necessary and must undergo an arteriovenous graft (AV graft) placement in order to have bloodstream access for hemodialysis.
"Most AV grafts that are placed for hemodialysis access are comprised of a synthetic material, which suffers from significant drawbacks including a high rate of infection, or a propensity for occlusion due to thrombosis and intimal hyperplasia," said Jeffrey H. Lawson, M.D., Ph.D., associate professor of surgery at Duke University School of Medicine and an author of the research. "Due to high complication rates, each AV dialysis graft requires an average of 2.8 interventions over its lifetime just to keep it functioning. Hence, there is a huge clinical need for a functionally superior, off-the-shelf, AV graft that suffers from fewer complications than current materials."
The research was conducted by scientists from Duke University, East Carolina University, Yale University and Humacyte, and was funded by Humacyte. Overseeing the research and senior author of the article was Laura Niklason, M.D., Ph.D., founder of Humacyte, and professor of anesthesiology and of biomedical engineering at Yale University. Niklason is a recognized authority in regenerative medicine for arterial engineering and was leader of the team that recently created a functioning rat lung in a laboratory.
"Not only are bioengineered veins available at the time of patient need, but the ability to generate a significant number of grafts from a cell bank will allow for a reduction in the final production costs, as compared to other regenerative medicine strategies," said lead author Shannon L. M. Dahl, senior director of scientific operations and co-founder of Humacyte. "While there is still considerable research to be done before a product is available for widespread use, we are highly encouraged by the results outlined in this paper and eager to move forward with additional study."
About The Research
In this research, bioengineered veins were generated in a bioreactor, decellularized, and stored up to 12 months in refrigerated conditions. The bioengineered veins (3-6mm in diameter) demonstrated excellent blood flow and resistance to occlusion in large animal models for up to one year.
For more information: www.humacyte.com

