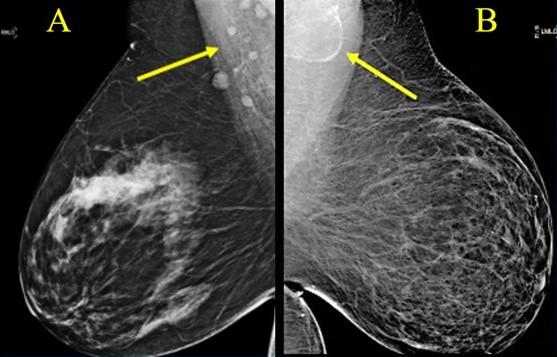
Variable LN morphology on screening mammograms in women with obesity due to ectopic fat deposition. A, Normal axillary lymph nodes measuring < 1.5 cm in 63-year-old woman with BM = 43.2. B, Fat-enlarged axillary node with large fatty hilum measuring 4.2 cm in 52-year-old woman with BMI = 45.8.
May 10, 2024 — According to the Summa Cum Laude Award-Winning Online Poster presented during the 124th ARRS Annual Meeting, fat-enlarged axillary nodes on screening mammograms can predict high cardiovascular disease (CVD) risk, Type 2 diabetes (T2DM), and hypertension (HTN).
“Incorporating fat-enlarged nodes into CVD risk models has the potential to improve CVD risk stratification without additional cost or additional testing,” said Jessica Rubino, MD, from Dartmouth Hitchcock Medical Center in Lebanon, NH. “Fat-enlarged axillary lymph nodes visualized on screening mammography may increase the ability to identify women who would benefit from CVD risk reduction strategies and more intensive risk assessment with coronary artery CT.”
Rubino et al. reviewed patients (women, 40–75 years) without known coronary artery disease who had a routine screening mammogram and cardiovascular risk factors available in the EMR within 1 year of the index mammogram (January 1, 2011–December 31, 2012). Evaluating major adverse cardiovascular events (MACE) within 10 years of the index mammogram, the researchers used clinical parameters at the time of the index mammogram to determine high estimated CVD risk via the pooled cohort equation (PCE) —defined by the American Heart Association as more than a 7.5% likelihood of MACE within 10 years. Two breast imagers evaluated screening mammograms to measure the length of the largest visible axillary LN in each breast in the mediolateral oblique view, analyzing the largest visible node for each patient. Logistic regression then examined associations between lymph node size, 10-year CVD risk, MACE, T2DM, HTN, low density lipoprotein (LDL), age, and BMI.
Ultimately, among 1,216 women included in this ARRS Annual Meeting Summa Cum Laude Scientific Poster, 907 (74.6%) had a visible axillary LN on the index mammogram, and 232 (19.1%) women had fat-enlarged nodes—defined as larger than 20 mm in length due to an expanded fatty hilum. Women with fat-enlarged nodes had a high risk of CVD defined by PCE (OR = 2.6, 95% CI 1.5–4.2), as well as a higher prevalence of T2DM (OR = 4, 95% CI 2.1–7.7) and HTN (OR = 2.5, 95% CI 1.6–4.0). Fat-enlarged nodes were also associated with a trend toward higher risk of MACE (OR = 1.7, 95% CI 0.9–3.1) and LDL (OR = 1.4, 95% CI 0.9–2.1).
“These results support further investigation of fat-enlarged lymph nodes,” Rubino added, “particularly with studies leveraging AI evaluation of mammographic fat-enlarged LNs and cardiometabolic disease.”
For more information: www.arrs.org


 February 18, 2026
February 18, 2026 









