May 3, 2022 — IBA (Ion Beam Applications S.A.), a leader in particle accelerator technology and the leader in dosimetry quality assurance solutions, today announces it has acquired Modus Medical Devices Inc. (Modus QA), a Canadian company based in London, Ontario, specialized in phantoms for quality assurance for radiation therapy.
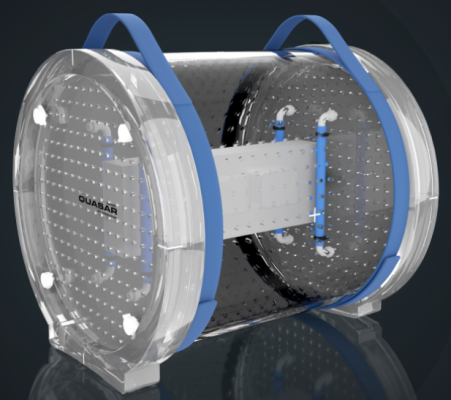
Founded in 2000 and employing about 20 people, Modus QA is at the forefront of quality assurance for complex radiation therapy technologies, such as image-guided radiation therapy (IGRT), surface guided radiation therapy (SGRT) and respiratory gating that have led to significant advances in patient treatment. It specializes in phantoms, a manufactured product made to resemble human organ or tissue used to quantify the potential effects of a radiation dose on a patient. The company has a solid installed base, with over 6,500 phantoms used in more than 4,000 clinics and hospitals worldwide. Built on science, research, development and collaboration with medical physicists, Modus QA is fully committed to delivering the accuracy needed to provide the best patient care.
Through this acquisition, IBA will benefit from synergies both in R&D and in sales and enable it to diversify its product catalogue. This should strengthen IBA’s competitive position globally as well as its footprint in North America. Thanks to the complementary products from Modus QA, IBA Dosimetry will be able to market the most advanced range of phantoms for radiation therapy quality assurance, strengthening its leading position in this segment. IBA will leverage its large client base to channel Modus QA’s products and, therefore, expand their reach to new markets in Europe and Asia.
Together, IBA and Modus QA will expand their product portfolio in medical imaging and in proton therapy and further strengthen existing relationships with Original Equipment Manufacturers (OEMs). In addition, within proton therapy, Modus QA’s expertise will enable IBA to develop new dosimetry products, in order to offer an end-to-end range of quality assurance products to proton therapy centers.
Jean-Marc Bothy, President of IBA Dosimetry, commented: “Modus QA are renowned experts in the dosimetry space and this highly complementary acquisition further enhances our world leading dosimetry offering. We are very pleased to welcome the Modus QA team’s expertise to the Dosimetry team, and are looking forward to working together to deliver IBA Dosimetry’s mission to treat even more patients safely.”
Olivier Legrain, Chief Executive Officer of IBA, added: “We welcome all Modus employees to the IBA family. This acquisition is not just important for the Dosimetry business, but is reflective of our wider company strategy to seek out value-enhancing business opportunities to build out our state-of-the-art product offering across our target markets.”
For more information: www.iba-worldwide.com


 February 04, 2026
February 04, 2026