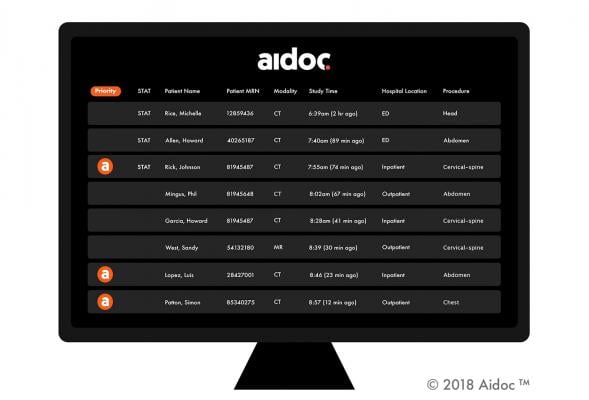
March 4, 2019 — Artificial intelligence (AI) radiology solution provider Aidoc announced the commercial release of its CE-marked product for the identification and triage of pulmonary embolism (PE) in computed tomography (CT) pulmonary angiograms. By flagging obstructions in blood-flow to the lungs, Aidoc prioritizes radiologists’ work queues and helps them detect critical conditions faster, leading to quicker treatment and saving lives.
In the United States alone, up to 600,000 people are diagnosed with PE annually and it is estimated to be responsible for 100,000 annual deaths, making it the third most common cause of cardiovascular death. PE diagnosis can be highly challenging due to its variable and non-specific presentation, making the case that it can truly benefit from AI-driven workflow triage.
At the 2019 European Congress of Radiology (ECR), Feb. 27-March 3 in Vienna, Austria, Aidoc ]demonstrated its suite of AI-based workflow solutions. The diagnostic performance of Aidoc’s PE solution at the University Hospital of Basel was presented as part of the ECR scientific sessions, demonstrating research concluding that Aidoc can complement conventional workflows with worklist prioritization and has the potential to improve the quality of healthcare by accelerating the diagnostic process and communication.
Read the abstract and watch the recorded ECR presentation here.
For more information: www.aidoc.com


 March 06, 2026
March 06, 2026