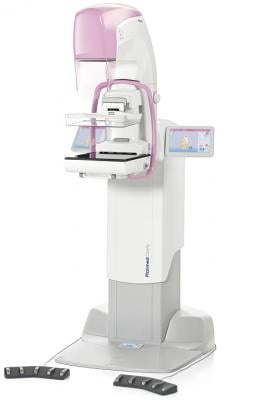
January 8, 2018 — The U.S. Food and Drug Administration (FDA) issued an approval letter for the Planmed Clarity 2-D full-field digital mammography (FFDM) system on Dec. 28, 2017, the company announced. The Planmed Clarity 2-D system is a digital mammography system that provides high image quality, optimal ergonomics and excellent usability for breast cancer screenings, according to the company. In addition to screening mammograms, the system supports diagnostic work-up examinations and biopsy procedures.
Planmed said the functional design of the unit guarantees optimal ergonomics for both the radiographer and the patient. The compact design relieves patient anxiety, and the flexible compression paddle adapts to patients of all sizes, making the patient experience comfortable and relaxed.
Additional features include customizable image processing options and a stable digital detector for working in extreme conditions, such as humid environments or mobile clinic installations.
For more information: www.planmed.com


 March 06, 2026
March 06, 2026 









