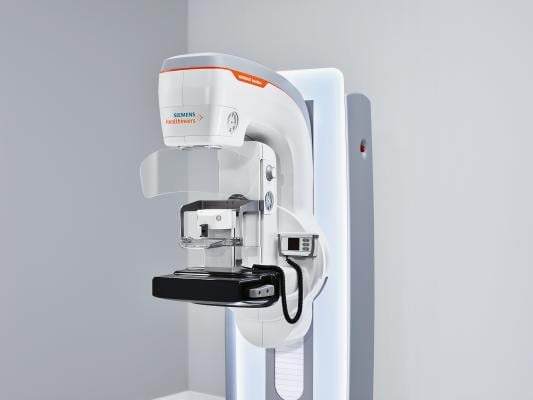
December 11, 2017 — Siemens Healthineers unveiled the new Mammomat Revelation premium mammography platform at the 2017 Radiological Society of North America (RSNA) Annual Meeting, Nov. 26-Dec. 1 in Chicago. Mammomat Revelation enables healthcare providers to expand precision medicine, improve clinical outcomes and increase patient satisfaction, according to the company. The system’s HD Breast Biopsy solution enables one-click targeting of suspicious areas with a +/- 1mm accuracy. The new integrated specimen imaging tool facilitates immediate control of the biopsy directly at the mammography system. Clinical workflow is improved by eliminating the need for a second imaging system and by reducing patient compression time. Mammomat Revelation also provides automated breast density measurements at the point of care. Additionally, it expands existing diagnostic options and provides patient access to functional breast imaging with contrast-enhanced mammography.
With its 50-degree 3-D HD Breast Tomosynthesis, Siemens Healthineers said the system offers the widest scan angle available. This scan angle is the basis for what it calls the industry’s highest depth resolution, which provides extremely high-quality 3-D images to increase diagnostic confidence and enable earlier detection of even subtle lesions. With Mammomat Revelation, the clinician also can perform biopsies based on 3-D HD Breast Tomosynthesis. The HD Breast Biopsy solution enables one-click targeting of suspicious areas with a +/- 1mm accuracy.
Upon acquisition, the tissue sample must be X-rayed to confirm a successful biopsy. In clinical practice today, samples are usually imaged on a second system or on a dedicated specimen scanner, usually in a different room. During this time, the patient remains under compression. Mammomat Revelation can substantially reduce discomfort and anxiety with the InSpect integrated specimen imaging tool. Biopsy samples can be imaged and visualized at the technologist workstation within 20 seconds without radiation exposure to the patient. The system improves the biopsy workflow, shortens patient compression time and improves the patient experience.
In addition, Mammomat Revelation is the industry’s first mammography platform to provide automated breast density measurements at the point of examination, according to Siemens. Currently, radiologists must estimate breast density visually during the image reading process, which typically occurs after the patient has left the facility. Patients with high breast density at higher risk of developing breast cancer often require additional workup to rule out breast cancer. Additional imaging procedures require patient callbacks, which can lead to uncertainty and concern. The information provided by Mammomat Revelation not only enables immediate, personalized risk stratification, but it also allows the facility to provide supplemental imaging while the patient is at the facility. Patients receive results faster, minimizing patient uncertainty and stress.
In some cases, purely morphological information from a mammogram or tomosynthesis scan may be insufficient for a precise diagnosis. For example, distinguishing scar tissue from new lesions in a post-surgical examination can be difficult. In addition to purely morphological information, clinicians may require functional information that can be obtained through contrast-enhanced imaging, which is usually performed using breast magnetic resonance imaging (MRI). Mammomat Revelation enables functional imaging directly on the mammography system. Since mammography systems are more widely available than MRI scanners, patient access to more specific breast imaging tools can be improved. And since contrast-enhanced mammography is still not widely utilized, the modular arrangement of the new system enables its expansion to include this and other functionality on an as-needed basis.
Finally, discomfort and pain in addition to related anxiety are key reasons why patients avoid mammography exams. With Mammomat Revelation’s Personalized Soft Compression, the breast compression process is softened and the compression force is automatically and individually adjusted. Coupled with ergonomic SoftComp Paddles, Personalized Soft Compression enables better breast positioning, more consistent image quality, and reduced patient discomfort.
Mammomat Revelation and Insight BD are U.S. Food and Drug Administration (FDA) 510(k)-pending and are not commercially available in the United States.
For more information: www.usa.healthcare.siemens.com


 March 06, 2026
March 06, 2026 









