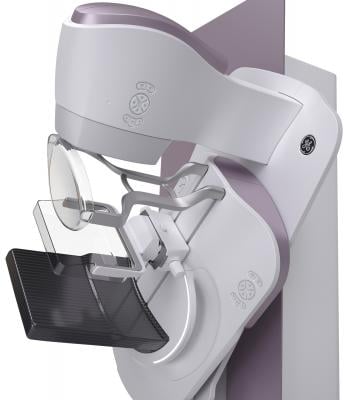
September 5, 2017 — The U.S. Food and Drug Administration (FDA) cleared the Senographe Pristina Dueta, a remote control device for GE Healthcare’s Senographe Pristina mammography system that allows patient self-compression. The device makes Senographe Pristina the first 2-D digital mammography system that allows patients to increase or decrease the amount of compression applied to their own breast before the mammogram X-ray is taken.
"Regular mammograms are an important tool in detecting breast cancer," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health. "However, some patients may experience anxiety or stress about the discomfort from the compression during the mammogram. This device allows patients some control over the amount of compression for their exam."
Mammograms can help detect breast cancer when it is in its early, most treatable stages. A conventional mammogram is a low-dose 2-D X-ray picture of the breast. Digital mammograms use a computer along with X-rays to make and show breast pictures, which are taken in the same way as a conventional mammogram. The resulting breast images are evaluated by a physician qualified under the Mammography Quality Standards Act (MQSA) to identify any abnormalities that might warrant additional work-up.
The Senographe Pristina with Self-Compression via Dueta is a digital mammography system designed to give the patient an active role in the application of compression. During a mammography exam, the technologist positions the patient and initiates compression. The technologist then guides the patient to gradually increase compression using the Senographe Pristina Dueta remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted.
The Senographe Pristina with Self-Compression was reviewed through the premarket notification 510(k) pathway. The FDA determined that the system is substantially equivalent to the predicate device Senographe Pristina. A clinical validation demonstrated that the addition of a remote to allow self-compression did not negatively impact image quality. Additionally, performing a mammogram with patient-assisted compression compared to compression solely applied by the technologist did not significantly increase the time of the exam.
For more information: www.gehealthcare.com


 March 06, 2026
March 06, 2026 









