March 3, 2015 — Calculating and tracking mammographic compression pressure could help standardize mammography quality and patient experience, according to a study published in the European Jorunal of Radiology (EJR).
The study, “Mammographic compression — A need for mechanical standardization,” compared compression between sites in the Netherlands and the United States using VolparaAnalytics from Volpara Solutions. Factors measured and compared included average force, pressure, breast thickness, breast volume, volumetric breast density and average glandular dose.
According to researchers, the lack of consistent guidelines regarding mammographic compression has led to wide variation in its technical execution. Breast compression is accomplished by means of a compression paddle, resulting in a certain contact area between the paddle and the breast, which can result in varying levels of discomfort or pain. On current mammography systems, the only mechanical parameter available in estimating the degree of compression is the physical entity of force (daN). Recently, researchers have suggested that pressure (kPa), resulting from a specific force divided by contact area on a breast, might be a more appropriate parameter for standardization. For example, while a large breast requires more force to compress and a small breast requires less force, research suggests that optimal compression pressure should be similar for all breast sizes.
This study compared the current compression practice in mammography between two sites in the Netherlands and one in the United States, and investigated whether the compression protocols could be improved by standardization of pressure (kPa) as an objective parameter. The Dutch dataset comprised 37,518 screening mammographic images (from 9,188 women) obtained from the Dutch national breast cancer screening program. The U.S. dataset comprised 7,171 screening and diagnostic mammographic images (from 1,851 women).
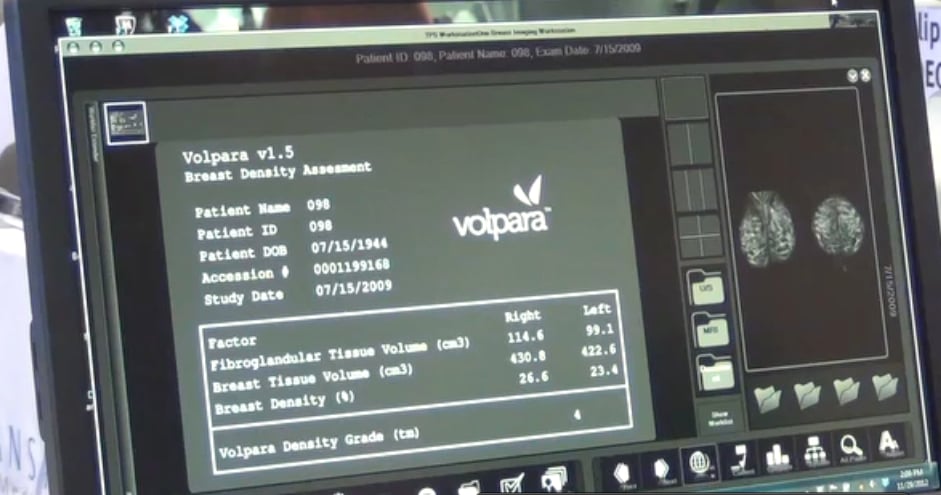
All images were analyzed using VolparaAnalytics and VolparaDensity software, so that the various factors could be compared between populations. Large variation in compression was observed for the Dutch and U.S. datasets, with relative standard deviations of 19.6 percent and 41.9 percent for force and 43.1 percent and 50.6 percent for pressure, respectively. Significant differences (p<0.001) were observed for the mean forces and pressures used in both the Dutch and U.S. datasets (i.e. 13.8 daN versus 7.4 daN and 13.7 kPa versus 8.1 kPa, respectively). As a result of the lack of standardization, extreme pressures were observed in both datasets, resulting in an inability to predict the compression pressure for a given individual.
“Now that software like VolparaAnalytics—which enables device-independent cross-comparisons of key mammographic metrics between patient populations—has become available, we may be able to better standardize mammographic compression, which could decrease variation and improve reproducibility; minimize unnecessary pain; and reduce radiation dose and inadequate image quality. I expect that this will enable the industry to enhance quality control of mammographic compression procedures and help maintain exam quality and patient comfort,” said Gerard J. den Heeten, LRCB Dutch Reference Center for Screening, Nijmegen, The Netherlands.
Cleared by the U.S. Food and Drug Administration (FDA), HealthCanada, the Therapeutic Goods Association (TGA) and CE-marked, VolparaDensity is in use at breast imaging centers worldwide to help radiologists objectively assess density from both digital mammography and tomosynthesis images, helping doctors evaluate who might benefit from additional screening. Highly correlated to breast magnetic resonance (MR) assessments, VolparaDensity automatically generates an objective measurement of volumetric breast density correlated to the American College of Radiology (ACR) breast density categories.
VolparaAnalytics is a centralized dashboard that produces configurable reports for management of quality assurance, resource utilization and breast imaging workflow. The software monitors critical elements of the breast imaging process and generates key metrics to help breast imaging centers identify and address potential issues early. VolparaDose provides a standardized measurement of patient-specific dose with consistent results across systems from different manufacturers.
For more information: www.volparasolutions.com


 February 18, 2026
February 18, 2026 









