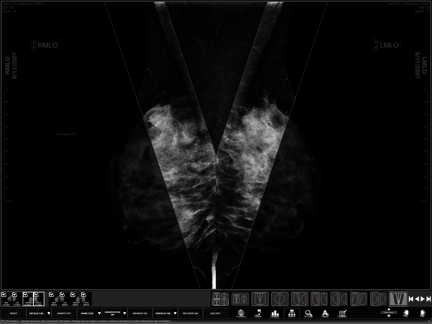
Screen Capture of a WorkstationOne showing the Systematic Masking step of the workflow
October 21, 2014 — Three Palm Software announced that that the U.S. Patent and Trademark Office has granted Three Palm Software a new patent, U.S. Patent 8,803,911. This new patent covers many aspects of the design of a mammography workstation, including pre-fetching and caching of the source data, sequencing of the hanging protocols, and advanced viewing techniques. In addition to common mammography viewing mechanisms such as current and prior comparisons, all-pixel viewing, and generation of reports, this patent also covers the “Systematic Masking” technique.
The mechanisms covered by this patent are implemented in the company’s flagship product — WorkstationOne. This patent, along with earlier patents that cover other aspects of mammography review such as tomosynthesis, demonstrates the leadership and strength of Three Palm Software in the digital mammography domain.
WorkstationOne includes all standard mammography tools such as screening and diagnostic workflows with user-selectable hanging protocol sequences, and it also provides advanced tools such as systematic masking and multi-view cross-correlation. At the enterprise level, WorkstationOne bundles bi-directional integration capabilities for picture archive and communications system (PACS), radiologic information system (RIS) and reporting systems. WorkstationOne also includes rich features for tomosynthesis display. All existing (more than four hundred) customized hanging protocols are fully available for both conventional mammography and tomosynthesis studies, enabling radiologists to read all mammography images efficiently using the built-in workflow.
WorkstationOne is sold worldwide by Three Palm Software’s partners.
For more information: threepalmsoft.com
Screen Capture of a WorkstationOne showing the Systematic Masking step of the workflow


 February 06, 2026
February 06, 2026 









