December 13, 2013 — Philips unveiled the Vereos PET/CT fully digital positron emission tomography/computed tomography (PET/CT) imaging system at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
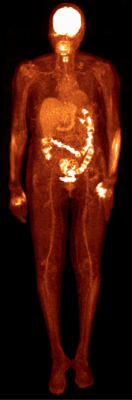
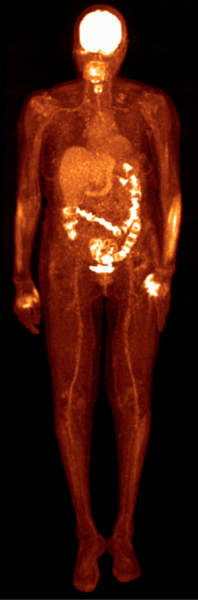
Based on Philips’ proprietary digital photon counting technology, the Vereos PET/CT is the first PET/CT system in the industry to use digital silicon photomultiplier detectors instead of traditional analog detectors, resulting in a step change in performance that approximately doubles sensitivity gain, volumetric resolution and quantitative accuracy compared to analog systems. These radical improvements can ultimately be translated into high image quality, increased diagnostic confidence, improved treatment planning and faster workflows.
In a recent survey, nine out of ten referring physicians preferred Vereos digital PET/CT images over images taken with an analog system. Philips has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its digital PET/CT system in the United States.


 February 27, 2026
February 27, 2026 









