Hologic is dedicating a significant share of its RSNA exhibit to the clinical benefits of 3-D
mammography in breast cancer screening and diagnosis as well as its recently introduced lower-dose 3-D mammography and 3-D guided breast biopsy capabilities.
Lower dose 3-D mammography
The C-View 2-D software option is designed to generate a 2-D image from a 3-D mammography data set without the need for separate 2-D X-ray exposures. C-View software is designed to offer radiologists and patients a number of benefits, including a reduction in X-ray exposures leading to shorter exam times (less than four second scans per view) and increased patient comfort from the shorter compression time. Hologic’s 3-D mammography exam using C-View software is a lower dose mammogram than the average U.S. 2-D mammogram, and yet it includes all the benefits of a combined 2-D and 3-D mammography exam.
3-D guided breast biopsy
The Affirm 3-D mammography biopsy option is designed for the localization and accurate targeting of regions of interest and is especially important for targeting lesions not detected in 2-D imaging or when using other modalities. This new biopsy technique is designed to provide numerous advantages over traditional stereotactic biopsy procedures, including faster lesion targeting and reduced patient procedure time. The Affirm system is preprogrammed for use with the Company's Eviva and ATEC vacuum-assisted breast biopsy devices.
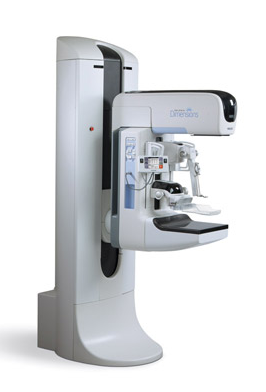
The Selenia Dimensions 3-D mammography system is currently the only U.S. Food and Drug Administration (FDA)-approved 3-D system in the United States, and Hologic is the only company worldwide that can provide a 3-D breast biopsy solution.
The 3-D mobile coach is making a special stop at RSNA on its yearlong, cross-country “3-D Technology: A More Accurate Mammogram” tour, which is dedicated to spreading the word about the important clinical benefits of 3-D mammography.
Additionally, over 60 new scientific papers, poster sessions and sponsored courses will be presented at RSNA on 3-D mammography, many of which used Hologic’s 3-D technology.
Other products:
- The ImageChecker CAD 10.0 software for C-View 2-D images will be on display and available for demonstration at RSNA 2013. The CAD C-View 2-D software is currently in review with the FDA.
- Single Energy (SE) Femur Exam capability is exclusive for the Hologic Horizon DXA system for the assessment of features associated with atypical femur fracture (AFF). This capability assists clinicians in their identification of potential AFFs in patients who have been on anti-resorptive treatments such as bisphosphonates. The 15-second SE Femur Exam is designed to produce a high-resolution image of the entire femur with a very low effective radiation dose. The Horizon DXA system was launched in August and is designed to offer clinicians expanded technical capabilities, workflow efficiencies and improved design components that assist clinicians in the assessment of critical health problems: osteoporosis, cardiovascular disease, body composition and atypical femur fractures.
- MultiView MR software offers advanced options for Hologic’s MultiView platform software. Hologic’s MR software is designed to provide real-time 4-D (3-D plus time) image processing, speed, flexibility and dedicated magnetic resonance imaging (MRI) algorithms for both breast and prostate diagnostic viewing.


 February 23, 2026
February 23, 2026 









