August 6, 2013 — Philips Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Pinnacle³ Proton treatment planning system.
Philips’ Pinnacle³ is a treatment planning system for external beam radiotherapy, which provides accuracy and reliability to users independent of the treatment delivery system. The Proton module is fully integrated into the Pinnacle³ planning environment and continues to offer personalized user experience.
"The transition for an existing and well-established photon center, to planning protons, is nicely facilitated by the Pinnacle³ system, whether it’s for proton-only planning, or combination planning of photons and protons. This capability has allowed us to confidently choose the best treatment modality for each patient," said Eric E. Klein, M.D., professor of radiation oncology, Washington University in St. Louis, Mo.
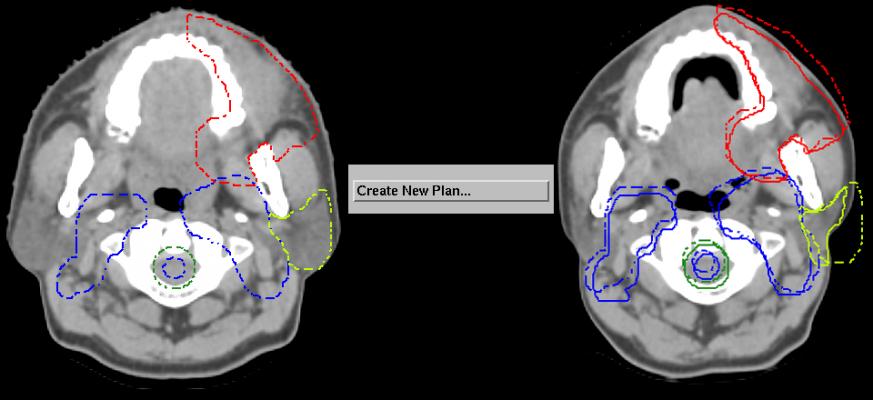
Designed through extensive clinical collaboration with current proton treatment centers, the Pinnacle³ proton module addresses some of the major challenges faced within proton planning today. The combination of specially designed tools and the seamless integration within Pinnacle³ provides an extensive range of functionalities that include composite proton-photon planning, fast commissioning and automated contouring and re-planning. These features offer clinicians the chance to select the appropriate treatment options for the patient and do the work quickly through improved workflow.
Features like automated re-planning allow the clinicians to adapt the treatment plans quickly (which, in turn, allow them to change the treatment regime) and take into account the impact of the treatment on the patient’s anatomy, providing more targeted treatments. Without these automation tools, the task remains very laborious and time-consuming; deterring clinicians from developing alternative treatment plans.
The number of centers treating patients with proton therapy is steadily increasing. With the introduction of more cost-effective treatment machines, which are reducing the investment cost of starting a proton center, the number of new programs under development is rising rapidly. Many of these centers see themselves as pioneers that advance the possibilities to provide improved care for cancer patients.
The system received FDA clearance in August 2013.
For more information: www.healthcare.philips.com


 January 30, 2026
January 30, 2026 









