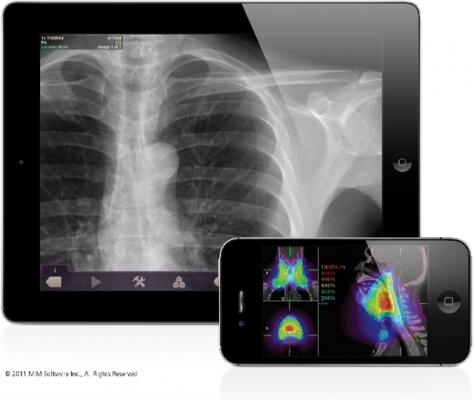
December 16, 2011 – MIM Software Inc. said Mobile MIM received its second U.S. Food and Drug Administration (FDA) 510(k) clearance for the release of its new version, Mobile MIM 3.0. Mobile MIM is now cleared for diagnostic X-ray and ultrasound viewing, as well as radiation treatment plan review and approval. Mobile MIM 3.0 is available on the Apple App Store. The app includes sample images to demonstrate its expanded functionality.
Radiation oncologists can use Mobile MIM to review dose volume histograms, isodose curves, contours and images for treatment plans – actions commonly restricted to a limited number of dedicated workstations.
“Mobile MIM provides a timesaving alternative to treatment plan review and approval by providing the necessary data and visualization for decision making while simultaneously reducing contention on costly treatment planning systems,“ said Jerimy Brockway, software director at MIM. Making Mobile MIM intuitive for radiation oncology was a primary goal, and we focused on creating the most natural and streamlined experience possible. The feedback so far has been overwhelmingly positive.”
Mobile MIM is capable of displaying very large images, more than 25 megapixels, with lossless data compression. It is a thick client that brings the data to the device for fast and consistent manipulation, independent of network performance. Device-level hardware encryption provides security for HIPAA compliance, and users have the additional security option of removing data from the device after viewing. For rapid access, images are displayed as they download. Data can be downloaded to Mobile MIM using MIMcloud or an on-site MIM workstation.
With the release of Mobile MIM 3.0, MIM Software also plans to launch a co-branded version of the app with its partner Accuray Incorporated. The co-branded Accuray version of the app, PlanTouch will have an interface that allows physicians to review and approve a CyberKnife treatment plan via a direct link.
MIM Software Inc. provides practical imaging solutions in the fields of radiation oncology, radiology, nuclear medicine, neuroimaging and cardiac imaging. MIM offers solutions for PC and Mac workstations, as well as mobile iOS and cloud-based platforms. MIM is a privately held company that sells its products globally to imaging centers, hospitals, specialty clinics, research organizations and pharmaceutical companies.
For more information: www.mimsoftware.com


 February 10, 2026
February 10, 2026 








