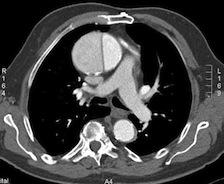
Aortic dissection (AD).
May 4, 2010 - Currently, using conventional imaging modalities to distinguish between acute and chronic aortic dissection (AD) for surgical risk evaluation is not possible. However, in a recent study, researchers were able to use fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) to detect reparatory hypermetabolism in the lacerated aortic wall of acute AD. This allowed them to determine if patients had acute or chronic stable AD.
Investigators at the Clinic for Vascular Surgery in Munich, Germany, analyzed the 18F-FDG uptake in the aortic wall of acute and chronic stable AD in 18 patients. Researchers analyzed the images qualitatively and looked at 18F-FDG uptake patterns and the standardized uptake values (SUVs) of the aortic wall, dissection membrane, and luminal 18F-FDG activity. The standard uptake value (SUV) ratio (maximum SUV in the aorta divided by mean SUV in the blood pool) was calculated to relativize individual luminal 18F-FDG spillover effects.
In contrast to chronic stable AD, all acute or acute progressive AD showed accentuated 18F-FDG uptake at the injured aortic wall or dissection membrane. The maximum SUV of the dissection membrane or aortic wall was significantly higher in acute AD than in chronic stable AD. Thereby, SUV varied from 3.03 to 4.64 (average maximum SUV, 3.84 ± 0.51) for the dissection membrane and from 2.22 to 4.60 (average maximum SUV, 2.94 ± 0.81) for the aortic wall, with false-negative and false-positive outliers. The discrimination between acute and stable AD was improved significantly, and false-positive or negative outliers were eliminated, using the SUV ratio method.
The results indicated 18F-FDG PET/CT might be useful in differentiating acute from chronic AD in clinically unclear cases. Researchers cautioned that larger studies are needed to confirm our preliminary results.
Reference: Reeps, C., Pelisek, J., Bundschuh, R., et al. "Imaging of Acute and Chronic Aortic Dissection by 18F-FDG PET/CT." The Journal of Nuclear Medicine. May, 1 2010; Vol. 51, No. 5
For more information: jnm.snmjournals.org


 February 03, 2026
February 03, 2026 









