May 21, 2010 – A second company is hoping to gain U.S. Food and Drug Administration (FDA) clearance for an optical coherence tomography (OCT) intravascular imaging system. Volcano Corp. said its second generation OCT system is pending FDA investigational device exemption (IDE) approval so it can start its VOILA clinical trial in the United States and South America.
Volcano received European CE mark clearance for its OCT system in January and plans the commercial release of the system in early 2011. The company hopes to release the system in the U.S. by mid-2011.
Lightlab received FDA clearance in early May for the first OCT intravascular imaging system approved in the United States. St. Jude Medical announced plans earlier this week it acquire Lightlab.
"St. Jude Medical's acquisition of LightLab confirms the validity and potential utility of this leading edge technology," said Scott Huennekens, president and CEO of Volcano. "We are planning a best-in-class OCT solution. We currently have CE mark in Europe on our first generation platform, and we are targeting a release of our second-generation platform in Europe early next year. We anticipate that this system will incorporate a smaller catheter, shorter prep time, faster pullback speed, and a longer imaging segment than the current device."
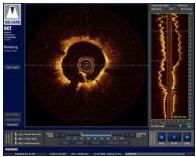
OCT provides high-resolution images of the vessel lumen and structural detail not seen with intravascular ultrasound (IVUS) or by angiography. With OCT, physicians can visualize structures in the lumen and the adjacent vessel wall down to a resolution of about 15 microns
"The Volcano OCT system provides fantastic images and is easy to use,” said Dr, Alex Abizaid from the Dante Pazzanese Cardiology Institute in Sao Paulo, Brazil With these OCT images, I can assess thrombus, lesion cap thickness, tissue growth on previously deployed stents and stent apposition at the time of implant. This is another great tool in addition to IVUS and FFR to help me augment angiography."
Volcano's integrated OCT platform will be built around the High-Definition Swept Source (HDSS), a nonlaser light source from Axsun Technologies, a wholly owned Volcano subsidiary. The HDSS has been tested at high speeds, which will translate into fast imaging and significantly faster pullbacks for the physician.
The new system is expected to be able to scan a lesion or stent length in as little as one second.
For more information: www.volcanocorp.com

