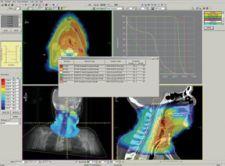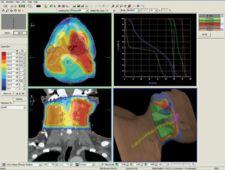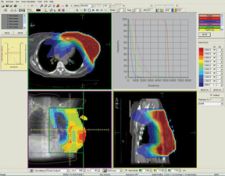
St. Louis, MO – CMS, the worldwide market leader in radiation treatment planning and workflow management solutions, has received FDA 510(k) clearance for Monaco, its next-generation IMRT planning platform. This clearance allows CMS to begin distributing Monaco for clinical use in the U.S. CMS previously announced the release of Monaco 1.0 in July and the first clinical implementations have already been completed in Europe and Australia.
Monaco represents a fundamentally new approach to IMRT planning. These concepts represent a unique and sophisticated set of tools to make the radiotherapy planning process easier, more straightforward and clinically reliable. The result is that clinicians are empowered with better tools to provide a higher standard of patient care. IMRT delivers significant improvements for radiation treatment and cancer patients, but the technology poses clinical challenges as well. A major challenge has been managing the time and resource-intensive processes associated with IMRT planning. With Monaco, CMS offers new approaches designed to improve the following:
• Repetitive trial-and-error methods for determining the optimal plan
• Multiple variables to manage the planning process including patient data, contours and the optimization itself
• A generally nonintuitive IMRT planning process
• A lack of control over the “black box” optimization process
Monaco was developed in collaboration with Markus Alber, Ph. D. and colleagues at the University of Tbingen in Germany. Monaco features innovative biological cost functions with multicriterial constrained optimization, a powerful leaf sequencer and a robust Monte Carlo dose calculation algorithm, and represents the most advanced IMRT solution on the market. Monaco is fully integrated with CMS’ Focal platform, providing seamless connectivity and integration with Focal’s advanced fusion, contouring, simulation and plan review capabilities, and represents the state-of-the-art for IMRT planning.
“We are pleased to receive clearance to distribute Monaco in the U.S. market,” said Andrew C. Cowen, President and Chief Executive Officer of CMS. “Monaco shows great promise in the efficiency and accuracy of radiation treatment
planning, and can positively impact the quality of care our clinical partners can provide to their patients.”
“Monaco comprises many new concepts. Over the years, CMS has proven to be a reliable and competent partner in developing these concepts into a highly useable clinical tool and a solid platform for future technologies,” said Markus Alber, Ph.D. “The ultimate goal of research should be its application in the clinic, and Monaco represents much more than a faithful translation of our work into a product thanks to the consistent and positive experience of CMS with treatment planning software.”
CMS is a worldwide leader in the development and support of radiation treatment planning and workflow management solutions. With treatment planning systems installed in more than 1,500 sites worldwide, CMS is a global resource to the radiation oncology community. A privately held corporation, CMS employs 290 professionals in its headquarters located in St. Louis, MO and regional offices in Tampa, FL; Freiburg, Germany; Tokyo, Japan; Sydney, Australia; and Shanghai, China.