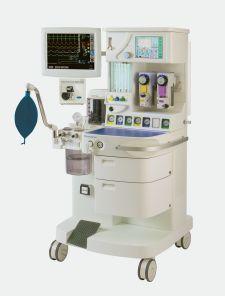
Oceanic Medical's Magellan family of anesthesia machines
Anesthesia depends on a constant flow of reliable physiological and respiratory gas data in order to properly manage a patient before, during and after a surgical procedure.
Sounds simple enough.
But in a profession known for reforming and policing itself after being rocked decades ago by malpractice lawsuits, machines that deliver anesthesia today have become reliable yet sophisticated and complex workhorses that have greatly restored the clinical community’s confidence in this age-old procedure.
From Liability to Reliability
Perhaps no single phenomenon shaped the anesthesia device market more than the civil lawsuit. And in the 1970s and 1980s, malpractice claims were literally flying at a clip that kept clinicians’ and administrators’ heads spinning.
Said one equipment manufacturer with whom Outpatient Care Technology spoke on the condition of anonymity, “During this time, a lot of the blame was being placed on the devices. Everyone was clamoring for new equipment because there was a lot of bad machines on the market.”
According to Thomas Barford, vice president, Anesthesia Systems, Patient Monitoring Division for Doylestown, PA-based Datascope Corp., in the closing years of the 20th century, the market was driven heavily by product and clinician liability. Out of that crisis the Anesthesia Patient Safety Foundation (APSF) was born. Just one year after it was founded in 1985, the group promulgated standards of care for its members, a first among any medical specialty. “To develop better technology standards for monitoring, the group pushed manufacturers to incorporate a lot of safety features,” Barford said. “As a result, accidents and liability claims started going down in the 1990s.”
While the number of claims are still uncomfortably high, the severity of adverse anesthesia events is lower today, according to Rob Clark, director of marketing for the perioperative business of Telford, PA-based Dräger Medical Inc. “The APSF did an analysis a few years ago of malpractice and liability claims in the 1970s, 1980s and 1990s and concluded that while significant improvements were made in patient safety, the number of claims were still high, though less severe,” Clark said.
Indeed, APSF proclaims that the number of deaths attributed to anesthesia has dropped to one in 400,000 from one in 10,000 for outpatient procedures in the past 20 years. The APSF credits its safety standards, as well as “today’s sophisticated equipment and improved medications and techniques,” to making anesthesia safer than ever before.
Today, the hot buttons at APSF are training, and like just about everywhere else, the electronic medical record. A recent study revealed a dearth of adequate training programs for new generations of complex anesthesia machines.
“New machines have unique and subtle variations in breathing circuit design, automated checkout, volatile agent delivery, hidden piston ventilators, fresh gas delivery, and ventilation modes,” researchers noted. Despite conventional pre-use instruction with, or without simulation, researchers concluded that anesthesiologists could not reliably assess their ability to safely use equipment in clinical practice. To their credit, many equipment manufacturers provide training on their machines, and Barford said he expects the APSF will develop standard training programs for the outpatient surgery and primary care arena within the next few years.
And it couldn’t come at a better time. According to a statement by the American Society of Anesthesiologists, “the strict, well-defined standards and regulations that help keep surgery and anesthesia very safe in hospitals and surgical centers do not uniformly apply to physicians' offices in the United States.” The ASA notes that only a few states and the District of Columbia currently require the same standards and regulations in doctors’ offices as they do in hospitals and surgical centers.
“Without minimum safety standards, however, there is a chance that office-based surgery may be taking place in environments with limited or outdated equipment, few or no emergency resources, inadequately trained staff or insufficient safety precautions,” the group said. To help remedy the situation, the ASA has developed comprehensive guidelines for office-based anesthesia and is urging states to adopt these same guidelines as regulations to protect all patients.
Some market analysts call the anesthesia machine market mature, but it continues to grow at a healthy clip thanks to the movement of many surgical procedures to the outpatient arena. According to researcher Medtech Insight, the total U.S. anesthesia and ventilation products market stands at about $1.9 billion, and is forecast to nearly double in size in just five years to $3.6 billion. “Sales of anesthesia delivery products such as integrated anesthesia systems, which combine anesthesia delivery and anesthesia monitoring, are expected to experience the most growth through 2012, followed by anesthesia and respiratory accessories, blood gas monitoring products, pulmonary function testing products, and ventilators,” the firm notes in a recent report.
Meanwhile, increasing competition and growing price sensitivity are fueling a wave of innovation, Frost & Sullivan notes in a 2005 report.
A ‘Wake-up’ Call
No single clinical development has shaped the anesthesia machine market more in the past decade than a phenomenon known as “level of consciousness” (LOC) monitoring. At some point during the malpractice crisis of the waning years of the 20th century, clinicians began gathering evidence that patients were “waking up” during surgical procedures; some even reported remembering the pain.
“There was no direct means of assessing a patient’s level of consciousness during surgery. For years, clinicians and researchers had searched for ways to translate the information from the electroencephalogram (EEG) into an assessment of a patient’s consciousness during surgery,” according to Norwood, MA-based Aspect Medical Systems, whose Bispectral Index (BIS) technology became the first clinically proven and commercially available direct measure of the effects of anesthetics and sedatives on the brain when it was launched in 1996. Using a sensor placed on the patient’s forehead, BIS monitoring translates information from the EEG into a single number that represents each patient’s level of consciousness.
BIS technology is one device success story. Today, Aspect claims that BIS technology is used in nearly a quarter of all U.S. surgical procedures requiring general anesthesia or deep sedation and in well more than half of all U.S. surgical suites. The BIS monitor is licensed for integration in Datascope, Datex-Ohmeda, Dixtal Medical, Dräger Medical, Nihon Kohden, Philips, Mindray and Spacelabs monitoring systems.
Some observers credit Aspect for selling the public as much as the Food and Drug Administration on the BIS. “Aspect has done a good job raising awareness about LOC and almost turned it into a consumer issue,” said Clark. Indeed, three years before Aspect gained FDA marketing clearance, USA Today reported a shocking clinical study that concluded anesthesia failure occurred 100 times each day in American operating rooms. The phenomenon wasn’t lost on Hollywood, either. The Internet Movie Database describes the plot of a 2006 “B-movie” horror film, appropriately called “Anesthesia,” this way: “A woman’s failed anesthetic during heart surgery leaves her fully conscious and able to feel all pain – but paralyzed and unable to warn anyone!”
Still, every manufacturer with whom Outpatient Care Technology spoke acknowledged LOC monitoring as one of the most significant innovations over the past 10 years. “Level of consciousness monitoring will be the largest growth area in the next few years,” declared Barford, who predicted that the number of LOC monitors will more than double between now and 2011. Indeed, Medtech Insight predicts new anesthesia monitoring technologies like LOC will lead growth in this market in the coming years.
But BIS has competition. For example, GE Healthcare has developed its own version of LOC technology with Entropy, which is now incorporated into the company’s line of anesthesia carestations, said Deb Schmaling, director of perioperative marketing for the Madison, WI, company. Schmaling notes that Entropy has garnered industry accolades, and it boasts a rapid response time (less than two seconds).
Aspect, too, has earned its share of industry praise, and approximately 2,800 published studies support a proven track record in practical applications. But surprisingly, LOC technology has yet to completely win over the anesthesiology community.
“Aspect did a great job lobbying and educating the public about the issue, but there’s still mixed reaction among physicians, and the jury is still out, I think,” said Clark. “You can measure the level of sedation and consciousness, but pain is another thing.” Moreover, a February 2007 study published by the ASA journal, Anesthesiology, concluded that anesthesia awareness rates might actually be much lower than previously reported (as low as 1 in 14,000 surgeries), particularly when continuous quality improvement methods are employed.
State of Innovation
While LOC monitoring may be the biggest development, it isn’t the only significant one. Manufacturers say the growth of outpatient surgery alone has literally transformed the industry. “The growth in demand from the surgery center market has resulted in more affordable machines that are still rich in features,” said Barford. In fact, Barford said that phenomenon alone actually pushed Datascope, a 43-year-old pioneer in patient monitoring, into the anesthesia delivery market. “We wanted to offer a total solution with monitors and delivery,” he said. “Hospitals looked at anesthesia machines as the mule that brings monitoring into the OR.”
Indeed, outpatient surgery has led to radical changes in surgical technique and device innovation, according to Clark. “The movement to less invasive procedures being performed in ambulatory surgery centers has also spurred innovation,” added Schmaling. “We’re also seeing higher volumes of procedures completed on spontaneous breathing patients (where paralytic drugs are not used and the patient maintains the ability to control respirations).”
Innovations also have allowed anesthesia machines to find their way into cath labs, endoscopy suites and MRI labs – places they never were before, observed Rod Peake, president of Atchison, KS-based Oceanic Medical. In fact, Peake learned firsthand of the need for MRI-friendly anesthesia machines while undergoing an imaging procedure. “The technologist told me they had a real need to sedate some patients undergoing the procedure because they were either overly anxious or quite heavy,” he said. That presented a unique opportunity to Oceanic, whose portable Magellan machine uses no ferrous metal parts.
The shift of surgeries to the outpatient arena also has led to procedures being performed on older, sicker and heavier patients, a phenomenon that placed significant demands on existing device technology, many manufacturers say.
“Five years ago, many patients in poor health or who were obese had to postpone surgeries until they were more stable,” said Clark. “Today, we’re able to operate on and maintain patients who are in a less-than-healthy state; this puts a great deal of additional stress on the ventilator.” Clarke noted that the number of bariatric surgeries in some centers has grown more than 40 percent in just the past two years. “This has placed greater demands for more powerful ventilators because bariatric patients generally have greater resistance and lower compliance to anesthesia,” he said.
These trends are so significant, in fact, that they led the ASA to revise its equipment obsolescence clause. “It used to be that a machine became obsolete and needed replacement when its safety requirements were no longer met,” Clark said. “Today, equipment is also deemed obsolete when it fails to meet the clinical requirements of the patient population for which it is intended.”
Schmaling said new modes of ventilation also are driving innovation, allowing caregivers to treat a new patient population in ambulatory surgery. Such modes, borrowed from their origins in intensive care, include pressure support ventilation, synchronized intermittent mandatory ventilation (or SIMV, in which assisted breaths are delivered at a set frequency with spontaneous breaths permitted in between delivered breaths), and pressure control volume guarantee.
In addition, device technology is being challenged by more and more spontaneous breathing techniques using laryngeal mask airways (LMAs), noted Russ Stearns, director of sales, anesthesia delivery and ventilation for Andover, MA-based Spacelabs Healthcare Inc. LMAs were introduced in 1991 as an alternative to the combination of face masks and other airway devices. “I think pressure support ventilation being available for use with laryngeal mask airways is a positive development,” added Justin Jeffries, marketing director for Louisville, KY-based DRE Inc. “Feedback regarding the other modes is very mixed. Some clinicians believe they add value, others are dismissive.”
Such developments also have spurred significant innovations in ventilator technology, leading to shorter recovery times and inpatient stays.
“While there has been some innovation in other areas of anesthesia equipment design, the area which has gained the widest acceptance by the users continues to be in the area of ventilation,” said Stearns. Barford added that multiple ventilation parameters are paving the way for more sophisticated machines: “In the old days, ventilators were very simplistic. [Anesthesiologists] just had to squeeze the bag to help the patient breathe.”
Finally, information technology is commonplace in most anesthesia machines today, thanks in part to efforts by the APSF to develop a benchmarking database to study anesthesia outcomes and Medicare’s fervent push for the entire healthcare industry to embrace the electronic medical record. In 2001, the APSF endorsed the use of anesthesia information management systems as a means of collecting data to create the national database. While APSF claims anesthesia information systems are installed in less that 5 percent of U.S. hospitals today, it expects more and more outpatient centers to look for machines that can easily transmit critical monitoring data to their information systems.
What Buyers Seek
What’s driving buying decisions in the outpatient arena? High technology at a low cost. “It’s a totally cost-driven market,” said Barford. “Outpatient surgery center buyers are looking for a low-cost system with the latest advanced features. They want more bang for the buck, so to speak.” It’s no surprise, therefore, that at the ASA annual meeting in October, Datascope plans to launch a new machine called the Navigator, which is based on giving surgery centers a high-end machine at an affordable cost (a $40,000 price point). Barford said Datascope hopes to deliver the first machines in January 2008.
Here’s a look at the major factors shaping buyer decisions:
• Low cost of ownership. Frost & Sullivan notes in its 2005 report that reducing the cost of ownership will be critical for manufacturers’ success in the anesthesia machine market. Everyone agrees. “Outpatient buyers know they are buying machines they won’t want to replace in just a few years,” said Clark, adding that machines are typically kept for 10 years or more.
• Low maintenance. While clinical innovations have been swift, manufacturers also are designing machines that not only are more durable (many tout fewer moving ventilator parts, for example), but also require less frequent maintenance. Some, in fact, now require only annual preventative maintenance visits, compared to the industry standard quarterly visit. “Many surgery centers don’t have biomedical departments, so maintenance issues are critical,” said Barford. And that has led to machines that are more reliable than ever before. “The impact of machine downtime can be huge, especially for smaller centers,” said Clark. “Most surgery centers don’t have backup anesthesia machines, and a typical center could lose $40,000 to $60,000 in surgery revenue each day an anesthesia machine is out of service.” When compared to a typical annual service contract that costs $1,000, the issue becomes a no-brainer. What kinds of things could go wrong with an anesthesia machine? “Computer chips could fail. Power supplies can fail. Components such as rubber materials need to be replaced because they can get brittle. It can get very expensive if you sit back and wait for the equipment to fail before making a decision,” said Clark. Added Schmaling, “There are an average of 2.5 ORs in a typical surgery center. If one anesthesia machine goes down, it can have a significant impact. Guaranteed uptime is very important.”
• Fewer proprietary disposables. Recent innovations have stemmed once mounting infection control concerns over cross-infections in such accessories as tubing and masks, and demand for disposables is growing. In fact, Frost & Sullivan predicts that demand will swell for anesthesia disposable products. Some machine makers encourage, and in some cases such as water traps and canisters, require buyers to use their line of disposables, something that is falling out of favor with buyers. Stearns said all of Spacelabs’ anesthesia machines use commonly used breathing circuits, disposable soda lime canisters and other user replaceable items rather than proprietary components, allowing customers “to access potentially lower cost operating supplies.” Clark acknowledges that proprietary disposables can be a problem for cost-conscious buyers. “When you look at the cost of the actual machine, it’s relatively low compared to the ongoing maintenance and consumables,” he said. “Buyers should analyze what consumables they’ll need over the life of the equipment and what it will cost the replace them.”
• Integration. “Surgery center buyers are looking for one system that does it all,” said Barford, “a single source for monitoring and anesthesia delivery.” Moreover, companies like GE Healthcare claim buyers are looking for one source for all their equipment needs, including disposables and maintenance. “We’re addressing the single source issue, which is big with ambulatory surgery centers,” said Schmaling, noting that GE Healthcare offers a full turnkey solution from planning and service to support and maintenance.
• Ease of use. With machines becoming more sophisticated, buyers are looking for machines that are easy to learn and use. “We designed our machines assuming that the guy operating it and taking care of it is a tech with a high school education – not a college educated engineer,” said Peake, whose company was recently awarded a $50 million contract to supply its portable and rugged Magellan-2200 anesthesia machine to the U.S. Army. “There are a lot of anesthesiology technologists operating this equipment in the outpatient arena so there is a great need for equipment to be reliable and easy to operate,” added Barford.