July 11, 2019 — Koios Medical announced its second 510(k) clearance from the U.S. Food and Drug Administration (FDA).
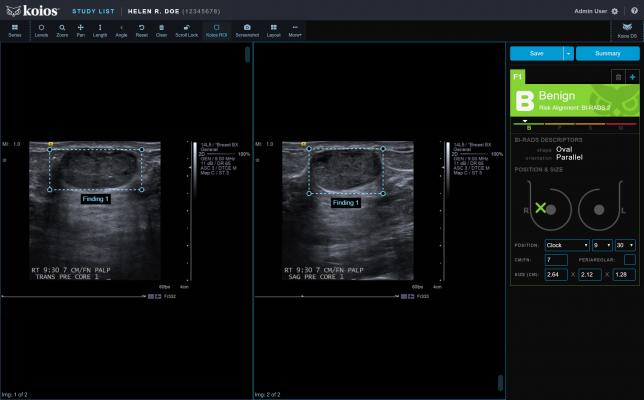
Koios DS (Decision Support) Breast 2.0 is intended for use to assist physicians analyzing breast ultrasound images. It aligns a machine learning-generated probability of malignancy with the appropriate BI-RADS (Breast Imaging-Reporting and Data System) category.
The software is cleared for use at the point of care, or connected to an image viewer for studies stored on picture archiving and communication systems (PACS). According to the company, Koios DS Breast 2.0 represents the most advanced artificial intelligence (AI)-based diagnostic technology for ultrasound image analysis to date. This patented software uses an ensemble of algorithms to aid the early detection of disease while also reducing biopsies of benign tissue.
In a recent reader study involving 15 physicians with relevant experience up to 39 years, each randomly analyzed 900 cases twice, separated by a one-month "washout period." Physicians utilizing the Koios DS 2.0 AI software experienced a statistically significant improvement in accuracy as measured by area under the receiver operating characteristic (ROC) curve, while simultaneously reducing both inter- and intra-operator variability.
Koios DS Breast 2.0 can be used in conjunction with most major PACS platforms and is directly available on the next-generation Logiq E10 digital ultrasound system from GE Healthcare. The Logiq E10 integrates AI, cloud connectivity, and advanced algorithms to acquire and reconstruct data. Machine-generated results can be exported directly into a patient's record.
Koios Medical continues to experiment with thyroid ultrasound image data and expects to add to its offering later this year.
For more information: www.koiosmedical.com
Related Content
Ways to Increase Productivity and Efficiency in Breast Imaging
Development of Artificial Intelligence in Women’s Health Emphasizes Value


 July 09, 2025
July 09, 2025