Image courtesy of JNM
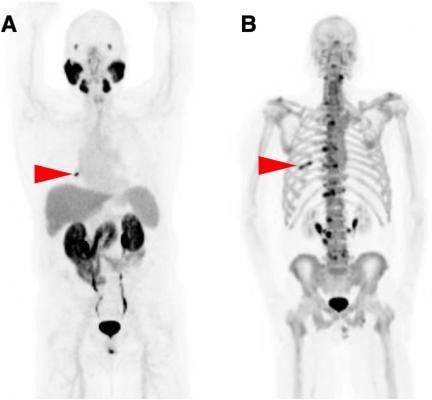
June 28, 2021 — The U.S. Food and Drug Administration (FDA) approved a new imaging agent for detection of prostate cancer, providing a more effective imaging approach to detect the spread of cancer to other parts of the body. Piflufolastat F-18 injection is the first fluorinated prostate-specific membrane antigen (PSMA) agent approved by the FDA and also the first commercially available PSMA PET imaging agent.
The agent is approved for PET imaging of PSMA-positive lesions in men with prostate cancer
- with suspected metastasis who are candidates for initial definitive therapy or
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
“We believe today’s approval is a game-changer for men facing prostate cancer,” said Jamie Bearse, Chief Executive Officer of ZERO – The End of Prostate Cancer, a patient advocacy group. “Having a diagnostic tool that allows doctors to see suspected metastatic or recurrent prostate cancer earlier, anywhere in the body, is a significant step forward and will have a tremendous impact on patients’ lives.”
The agent, initially called 18F-DCFPyL, was developed by Martin Pomper, MD, PhD, director of nuclear medicine and molecular imaging at the Johns Hopkins University School of Medicine. The New Drug Application for the agent was filed in September 2020 and granted priority review by the FDA in December. Research supporting the approval of the agent came from two pivotal multicenter studies—the CONDOR study and the OSPREY study.
The agent is manufactured by Lantheus as Pylarify injection and will be widely available throughout the United States.
For more information: www.snmmi.org
Related Prostate Imaging Agent Information:
PSMA-Targeted Radiotracer Pinpoints Metastatic Prostate Cancer Across Anatomic Regions
For Additional SNMMI21 Content:
PSMA PET/CT Can Change Management in Recurrent Prostate Cancer
PSMA PET/CT Can Change Management in Recurrent Prostate Cancer
Total-body Dynamic PET Successfully Detects Metastatic Cancer
New PET Radiotracer Proven Safe in Imaging Malignant Brain Tumors
Targeted Radionuclide Therapy Enhances Prostate Cancer Response to Immunotherapies
New PET/MRI Approach Pinpoints Chronic Pain Location, Alters Management


 May 03, 2024
May 03, 2024