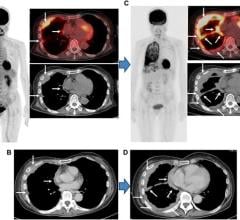
February 21, 2018 — Blue Earth Diagnostics announced that Axumin (fluciclovine F 18) injection has been added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Prostate Cancer (Version 1.2018). These updated NCCN Guidelines state that F-18 fluciclovine positron emission tomography/computed tomography (PET/CT) or PET/magnetic resonance imaging (MRI) scans should be considered in the clinical workup of patients with recurrence or progression of their prostate cancer. This recommendation is based on uniform NCCN consensus that the intervention is appropriate.
Axumin is a novel molecular imaging agent indicated for use in PET imaging to identify suspected sites of prostate cancer recurrence in men who have elevated blood levels of prostate specific antigen (PSA) following prior treatment. It is the first FDA-approved F-18 labeled PET scan agent indicated in patients with suspected recurrent prostate cancer.
“The NCCN Guidelines are widely used by clinicians and healthcare providers as a benchmark to assess clinical utility, and this update recognizes the important diagnostic ability of PET imaging to detect and localize the sites of recurrence in men with biochemically recurrent prostate cancer,” said Gerald L. Andriole, M.D., the Robert K. Royce Distinguished Professor and chief of urologic surgery at Washington University School of Medicine in St. Louis. “Many treatment options exist for these men and when PSA levels rise after primary treatment, knowing where the disease has recurred is essential to making appropriate patient management decisions. PET imaging can provide reliable information for these patients."
“Referring physicians need actionable information to guide patient management decisions,” said Lale Kostakoglu, M.D., MPH, professor of radiology and chief of nuclear medicine and molecular imaging, Icahn School of Medicine at Mount Sinai, New York, N.Y. “A PET imaging agent such as Axumin that can reliably detect and localize recurrent prostate cancer is important, due to the limited ability of other currently used imaging procedures to identify the extent and localization of recurrent disease in these patients.”
Axumin received Transitional Pass-Through Status in the Hospital Outpatient Prospective Payment System 2017 Final Rule from the Centers for Medicare & Medicaid Services (CMS), effective since Jan. 1, 2017. This also provides a product-specific A-code (A9588) for use with Medicare and private insurer patient claims. Axumin is currently available from 18 radiopharmacies throughout the United States, with additional manufacturing sites planned for 2018.
Prostate cancer is the second leading cause of cancer death in men in the United States. While most primary prostate cancer can be successfully treated, recurrence occurs in up to one-third of patients. Recurrent disease is typically detected by a rise in PSA levels, but often the location and extent of the disease cannot be detected by conventional imaging. Of those patients who experience biochemical recurrence, approximately one-third go on to develop metastatic prostate cancer.
For more information: www.blueearthdiagnostics.com


 May 30, 2025
May 30, 2025