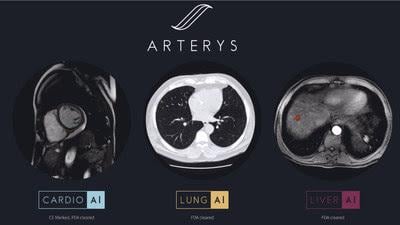
February 15, 2018 — Arterys Inc. announced its fifth 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Arterys Oncology AI Suite, an artificial intelligence (AI)-based, cloud-based medical imaging software.
The new oncology software complements Arterys’ existing web-based offering, and helps clinicians measure and track tumors or potential cancers, and easily apply radiological standards. The initial deep learning workflows will be for liver magnetic resonance imaging (MRI) and computed tomography (CT) scans, as well as for lung CT scans. With this new technology, radiologists can now easily confirm, evaluate, quantify, and report on the absence or presence of lung nodules and liver lesions along with their key characteristics using a simple web browser. The company plans additional deep learning workflows for solid tumors in other organs.
"The evaluation of primary and metastatic disease in the lung and liver are among the most valuable contributions of radiologists to the care of patients with cancer," said radiologist and Arterys co-founder Albert Hsiao, M.D., Ph.D. "We desperately need more efficient technology to automatically track lung and liver lesions to further improve diagnosis, assess response to treatment, and automate reporting with standardized terminology including Lung-RADS and LI-RADS."
Oncology AI runs on the Arterys MICA (Medical Imaging Cloud AI) platform, which the company said is easier to deploy than on-premise imaging systems and complies with patient data privacy and security requirements in 27 countries. The software uses deep learning to automate the segmentation of lung nodules and liver lesions, with accuracy equal to segmentations performed manually by experienced clinicians. The clinician has the capability to edit these automated segmentations, so they always remain in control.
For more information: www.arterys.com
Related Artificial Intelligence Content
Arterys Receives FDA Clearance for Arterys MICA Web-Based Medical Imaging Analytics Platform
Arterys Partners with GE Healthcare to Launch Viosworks Cardiac Imaging Platform
Arterys Showcases FDA-cleared 4D Flow MRI Software at RSNA 2016
Arterys Cardio DL Cloud MRI Analytics Software Receives FDA Clearance
VIDEO: Editor’s Choice of Most Innovative New Technologies at RSNA 2016


 July 09, 2025
July 09, 2025