November 20, 2018 — Stryker's Advanced Guidance Technologies business (formerly known as Stryker's Navigation business) has entered into strategic partnerships with Synaptive Medical and Ziehm Imaging, strengthening Stryker's position in the surgical guidance market.
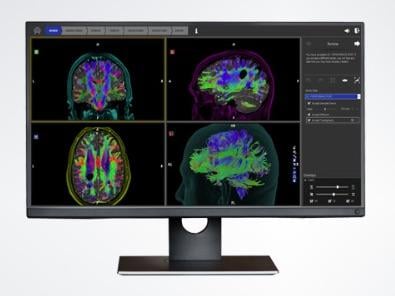
Through the partnership with Synaptive Medical, Stryker will offer the BrightMatter Plan software, which automatically generates high-fidelity, dynamic, whole-brain tractography. Surgeons can easily review tracts and explore multiple surgical approaches to create powerful pre-operative plans for every case. Tractography can be used, without disrupting surgical workflow, to confirm the data is concordant with the intended approach and determine whether detailed surgical planning is required. Then, the surgical plans can be easily exported for navigation in the operating room.
BrightMatter Plan is the only tractography offering, according to Synaptive Medical, that is automated and whole-brain. Other solutions on the market require a neuroradiologist or other tractography expert to generate tracts, which is both time consuming and a road block for surgeons.
The Ziehm Vision RFD 3D C-arm, which Stryker will now offer as part of its agreement with Ziehm Imaging, offers the latest flat-panel technology for computed tomography (CT)-like image quality. This gives surgeons access to detail-rich imaging that provides the accuracy and efficiency required in demanding orthopedic, trauma or spinal procedures, all with minimized dose and less time.
For more information: www.stryker.com


 April 23, 2024
April 23, 2024