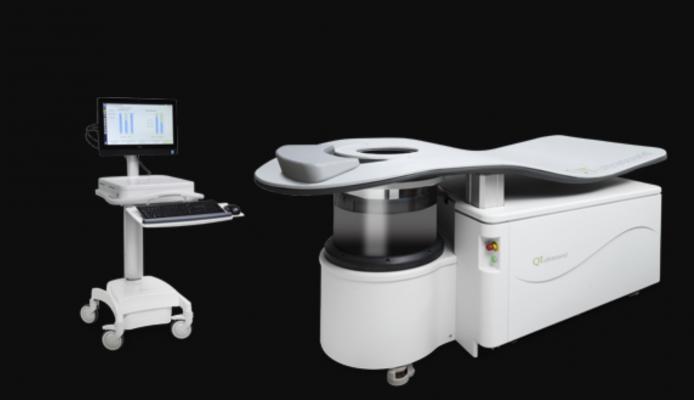
January 30, 2019 — QTbreasthealth launched a center in Grand Rapids, Mich., featuring its quantitative transmission ultrasound technology known as the QTscan. The U.S. Food and Drug Administration (FDA)-cleared 3-D ultrasound breast imaging technology provides women an option with no radiation, no compression and no injections. It also provides unimpeded visibility for women with dense breast tissue. QTbreasthealth’s goal is to provide technology that encourages women to get scanned as often as necessary, with no risk.
The Grand Rapids center is the third QTbreasthealth location, following two California locations in Marin and Walnut Creek, with several more locations scheduled to open around the country throughout 2019. News of the Grand Rapids opening comes on the heels of ongoing clinical trials, which have found the QTscan to be successful in identifying various types of breast tissue, particularly within dense breasts.
The new Grand Rapids center, like all QTbreasthealth locations, offers a spa-like breast imaging experience, complete with soothing scents, comforting robes and a relaxing imaging scan that some women actually sleep through. Unlike most other breast imaging centers, QTbreasthealth patients receive results via phone or teleconference with a provider within 72 hours.
Because the QTscan uses no radiation, the exam is available to women of all ages and avoids the frequency limits associated with radiation-based imaging tests. The clear and comprehensive, 3-D image delivered by the QTscan also makes QTbreasthealth an ideal second opinion for breast imaging, providing a more detailed image than mammography or handheld ultrasound.
For more information: www.qtultrasound.com

 April 24, 2024
April 24, 2024 








