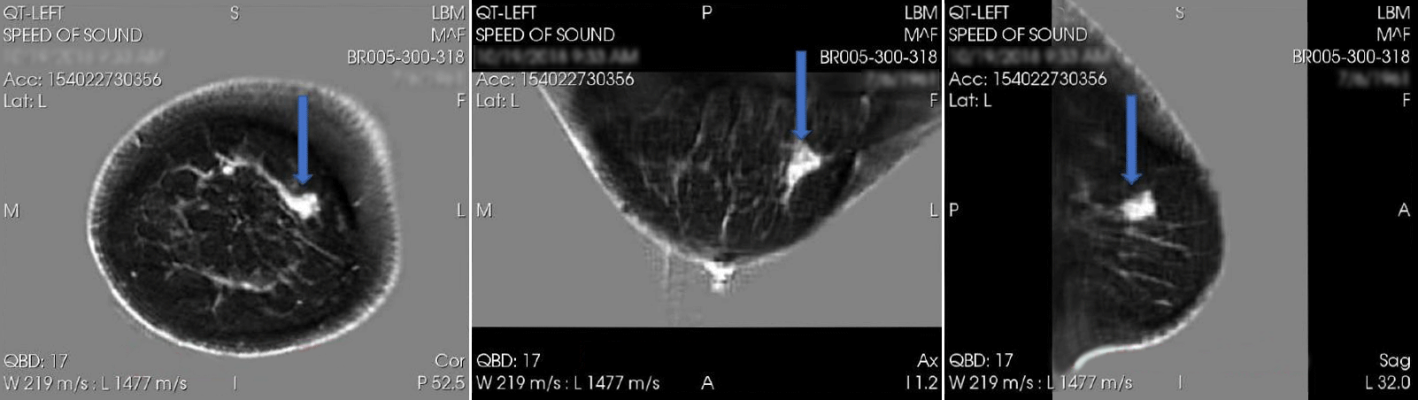
April 16, 2024 — QT Imaging Holdings, Inc., a medical device company engaged in research, development, and commercialization of innovative body imaging systems, announced positive data regarding the diagnostic performance of QTI’s Breast Acoustic CT Scans for mass detection from its second blinded multi-reader multi-case study. The study was published in Academic Radiology, online, in January 2024: A Multireader Multicase (MRMC) Receiver Operating Characteristic (ROC) Study Evaluating Noninferiority of Quantitative Transmission (QT) Ultrasound to Digital Breast Tomosynthesis (DBT) on Detection and Recall of Breast Lesions - Academic Radiology
Twenty-four breast radiologists participated in a study of 177 selected cases (66 with cancer, atypia, or solid mass and 111 normal or with nonsolid benign abnormality). The study found that QTI’s Breast Acoustic CTTM is similarly effective as digital breast tomosynthesis (DBT), also known as 3D mammography, in that the area under receiver operating characteristic curve (AUC) was statistically non-inferior for QTI scan compared with DBT for the AUC difference margin of -0.05. This means that QTI technology is non-inferior to DBT in the detection of breast lesions as a whole, with high specificity in determining benign cysts and thus decreasing benign recall rates. Statistical analysis was performed by Dr. Yulei Jiang from the Department of Radiology of the University of Chicago.
While fewer than 5% of women with breast cancer are diagnosed before the age of 40(1), those cancers are usually aggressive, and the young patients suffer from poor survival outcomes. Unfortunately, routine screening mammograms are not recommended for women under 40 because risks outweigh potential benefits at this young age. Fortunately, the now published results suggest that QTI’s technology can be a much-needed potential alternative to mammography for women too young to undergo mammography screening.
“We are encouraged by the consistent high performance of QTI’s technology, especially in women with dense breasts. Along with the previously published results and from comparison of QTI’s scans with mammography, these trial results will be valuable for our planned FDA submission for a screening indication in younger women identified with above-average risk for developing breast cancer and who have not yet reached the age for conventional mammography-based screening,” said Dr. Bilal Malik, Chief Science Officer. The results of the study comparing QTI’s technology performance with mammography were published in Academic Radiology, in December 2020: An Exploratory Multi-reader, Multi-case Study Comparing Transmission Ultrasound to Mammography on Recall Rates and Detection Rates for Breast Cancer Lesions - Academic Radiology
According to Jennifer Simmons, MD, Breast Surgeon, and Integrative Oncologist at Real Health MD, “Accurate and safe breast cancer screening is crucial for the 40% of women in US with dense breasts. Standardization and broad accessibility of any technology are essential to minimize the financial and emotional impacts of unnecessary biopsies and call backs. This study suggests that these requirements are now being met.” Dr. Simmons, who has been caring for women with breast disease for over 20 years, knows the increasing need for safe imaging in the younger population. “Cancers are occurring in much younger women today than when I started to practice. We need a safe imaging solution for young women. I am hopeful that QTI’s technology is that solution.”
For more information: www.qtimaging.com


 December 04, 2025
December 04, 2025