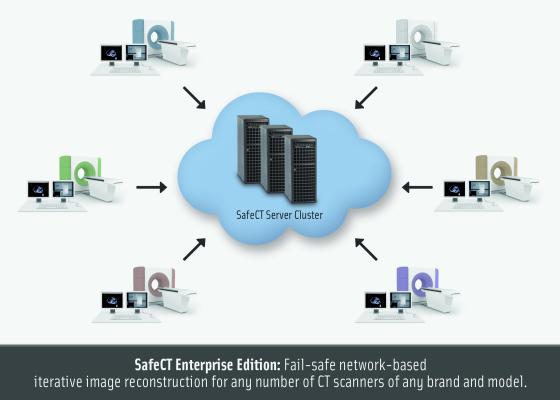
November 10, 2016 — Medic Vision Imaging Solutions Ltd. announced recently that the company has tripled its growth. Fueled by compliance regulations such as XR-29, low-dose computed tomography (CT) lung screening and CT dose reporting, demand for the company’s SafeCT suite of products is at an all-time high, it said.
Medic Vision's SafeCT product suite provides compliance with the latest regulatory requirements and initiatives related to CT radiation. U.S. Food and Drug Administration (FDA)-cleared SafeCT products are in routine clinical use at more than 150 major hospitals and imaging centers nationwide, supporting CT scanners of all vendors and models.
Recent successes include the FDA-cleared distinction for the SafeCT-29 product and:
- Philips Healthcare’s decision to recommend Medic Vision’s SafeCT-29 solution to all of its customers that have CT or positron emission tomography (PET)/CT scanners that cannot be updated to become fully compliant with XR-29 standard;
- RadNet, a national provider of high-quality, cost-effective diagnostic imaging services, selected the SafeCT product suite to enable compliance with low-dose CT scanning and XR-29 regulations for its facilities nationwide; and
- Numerous partnerships with leading providers of products and services including Southwest Medical Resources (Ontario, CA), Nationwide Imaging Services (Wall, N.J.), Radiology Oncology Systems (San Diego, Calif.), Medical Outfitters (Miami, Fla.) and others.
“This upgrade to our existing scanners with SafeCT Enterprise and SafeCT-29 from Medic Vision allows us to optimize patient safety while complying with regulations and staying within budget constraints,” said Howard Berger, M.D., president and CEO of RadNet. “Medic Vision’s products enable us to comply with new dose regulations with our existing scanners and, in the process, alleviate the need for us to replace a portion of our CT fleet. These Medic Visions products are FDA-cleared, giving us reassurance that we are complying with mandated government regulations.”
“Owners of non-compliant CT scanners in the U.S. still have three months before the 15 percent Medicare/Medicaid reimbursement penalties take effect, and we expect that the demand for SafeCT-29, the only FDA-cleared solution, will greatly increase,” said John Vano, president of Radiology Oncology Systems. “We believe Medic Vision has the best solution currently available and we are excited to be a part of the mission to help imaging centers become compliant."
For more information: www.medicvision.com


 April 23, 2024
April 23, 2024