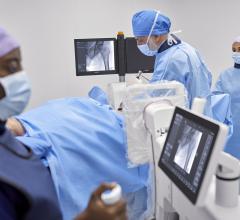
September 7, 2023 — The groundbreaking AURA 10 specimen imager by Belgian MedTech pioneer XEOS has been cleared by the FDA. The AURA 10 is now poised to help enhance surgical outcomes in the United States.
First-Ever PET-CT Specimen Imager
The AURA 10 marks a historic milestone for medical imaging in the United States, as the specimen imager is the first-ever PET-CTfor the operating room (OR) cleared by the US Food and Drug Administration (FDA). This revolutionary device presents surgeons and imaging specialists with an unprecedented level of precision, harnessing the sensitivity of PET imaging with submillimeter spatial resolution. The AURA 10 mobile scanner ensures that high-quality specimen images are captured right there in the OR, a mere 10 minutes after excision, effectively eliminating the need for specimen transport to the radiology or pathology departments during surgery. This advancement promises to transform the landscape of intraoperative diagnostics, offering real-time insights that can make all the difference in patient care.
Recognized as one of the most demanding regulatory pathways globally, the approval process is overseen by the FDA to ensure medical devices are safe and effective when introduced into the United States. It demonstrates that a company is able to deliver compliant and value-added solutions, based on adherence to stringent legislative requirements, which involve a detailed analysis of both technical and clinical data. Receiving FDA approval means that a device has a sound scientific foundation and also testifies to the fact that it genuinely enhances patient health and well-being.
“The FDA approval is a momentous achievement that underscores our commitment to propel healthcare forward through cutting-edge imaging solutions,” says Roel Van Holen, CEO of XEOS. “The AURA 10, a game-changing device born from a decade of molecular imaging technology research and development, is now authorized for the US market, following its successful introduction in Europe in 2022."
The Right Device for a Demanding Environment
Conventional PET/CT imaging plays a pivotal role in diagnosis and follow-up for a variety of medical conditions. However, there is a growing challenge in healthcare systems worldwide, as an increasing volume of complex patients puts pressure on intraoperative efficiency, which impacts the operating room but also other departments, such as radiology and pathology. Rapid verification of excised specimens during surgery is therefore of the utmost importance. The AURA 10 helps surgeons take informed decisions right on the spot by harnessing the power of molecular imaging. Pilot studies in various cancer types (including breast cancer, prostate cancer, cander of the head and neck region and many others) show promising results in terms of diagnostic accuracy.
“We are actively working with healthcare institutions across the United States to introduce the AURA 10 into their workflows, with the aim of improving patient care, clinical confidence and surgical efficiency,” says Vincent Keereman, CMO at XEOS. “The operating room faces enormous challenges in terms of patient volume and complexity. We aim to alleviate these pressures. The innovative technology of the AURA 10 assists surgeons in acquiring an ideal image for their purposes and analyzing it immediately in the right way. This may significantly reduce the time required for surgical assessment and prevent the need for additional procedures. Beyond its precision and time-saving capabilities, the AURA 10 also has the potential to extend peace of mind to patients by helping to ensure that surgery reaches its target. It reassures patients that they are taken care of in the right manner.”
For more information: www.xeos.care


 April 24, 2024
April 24, 2024