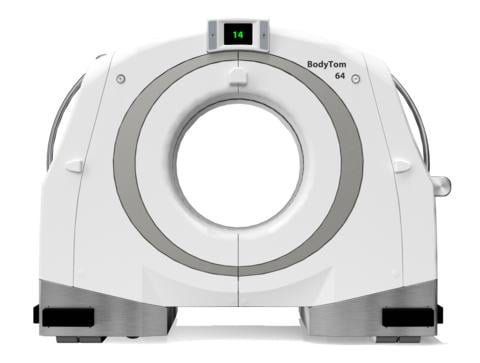
Designed to provide users with a multi-departmental imaging solution, the BodyTom 64 combines innovation and power to elevate the diagnostic experience. (Photo: Business Wire)
November 30, 2022 — NeuroLogica Corp., a subsidiary of Samsung Electronics Co. Ltd., announced that its head-to-toe trauma imaging solution, the BodyTom 64 Point-of-Care Mobile Computed Tomography (CT) Scanner, has received 510(k) clearance from the U.S. Food and Drug Administration for commercial use in the United States.
“We’re thrilled to build off our expertise and elevate point-of-care imaging with our BodyTom 64, which can transform any room in a hospital into an advanced imaging suite,” said Jason Koshnitsky, Sr. Director of Global Sales and Marketing of NeuroLogica. “This full-body 64-slice CT scanner is an upgraded version of the BodyTom Elite CT scanner, providing enhanced functionality with the same high-resolution imaging capabilities.”
Based on customer feedback, the company designed the BodyTom 64 to enhance the user experience and improve clinical workflows through revisions to both the software and the data acquisition system (DAS). Such revisions include incorporating Linux as the operating system and having the ability to generate up to 64 cross-sectional CT images of a patient’s body, versus the 32 images produced by the predicate BodyTom Elite.
With indications for both pediatric and adult imaging, the BodyTom 64 is a multi-departmental imaging solution that can be utilized for various needs, including:
- Neurosurgery/Surgery: When combined with any radiolucent skull fixation device, the BodyTom 64 can transform an operating room into an intraoperative neuro-imaging suite to enhance neuro-navigation and surgical outcomes, including clinical utility for extracranial procedures.
- Trauma/ER: The BodyTom 64’s unique combination of internal lead shielding and battery operation allows any standard trauma bay to be transformed into an advanced CT imaging suite.
- Interventional Radiology: BodyTom 64 can help optimize workflows by remaining ready to rescan for each stage of needle guidance, and bring the power of multi-slice CT to the interventional suite.
The BodyTom 64 brings the power of innovative imaging to patients’ bedsides safely and efficiently. BodyTom 64 is designed and manufactured to comply with the FDA Quality System Regulations and ISO 13485:2016 requirements and is in conformance with global regulatory harmonized standards.
For more infomation: www.NeuroLogica.com


 April 25, 2024
April 25, 2024