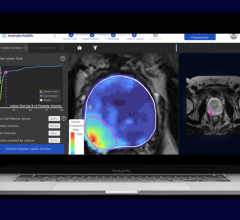
June 22, 2021 — At the Society of Nuclear Medicine and Molecular Imaging’s (SNMMI) 2021 summer conference, GE Healthcare showcased new molecular imaging products and solutions as well as new opportunities to expand access to radioactive tracers and other pharmaceutical imaging agents. This increase in access to the latest and most innovative precision diagnostics and radiopharmaceuticals – particularly with recent FDA clearances of critical new therapies – can provide clinicians unique opportunities to make personalized care decisions and treatment response assessments that may help improve patient outcomes.
As an example, GE Healthcare is doubling its U.S. distribution footprint of Vizamyl (flutemetamol F18) following the FDA's recent approval of Aduhelm (aducanumab), the first approved treatment for the reduction of beta-amyloid plaques associated with Alzheimer’s disease. This will significantly expand the availability of Vizamyl – a PET radiotracer used to diagnose the presence and density of beta-amyloid plaques in patients with suspected Alzheimer’s disease or other causes of cognitive decline – to imaging centers and clinicians. GE Healthcare will also launch education and awareness initiatives for patients and their families, clinicians, payers, and policymakers about the role amyloid imaging has in the diagnosis and monitoring of Alzheimer’s disease.
“For years we’ve been talking about precision health and theranostics as ‘the future of healthcare.’ Now, we are seeing it become a reality,” said Jean Luc Procaccini, President & CEO, Molecular Imaging & Computed Tomography, GE Healthcare. “Fortunately, GE Healthcare is ready, already having spent years developing cutting-edge products with precision health and theranostics in mind. From cyclotrons and chemistry synthesis to imaging solutions to radioisotopes and pharmaceutical diagnostics, we are proud to offer the tools and information needed by clinicians to make more personalized care recommendations. Afterall, healthcare is personal – our treatments should be too.”
Historically, medical treatments have been designed for the “average patient.” As a result of this “one-size-fits-all” approach, treatments can be very successful for some, but not for others. Precision healthcare or personalized medicine, on the other hand, is an innovative approach that uses one’s personal information and biomarkers — particularly genes, environments, and lifestyles — to help tailor treatment recommendations for individual patients. This approach to healthcare has in turn given rise to theranostics, a new field of medicine that allows for targeted therapies because of clinically precise diagnostic tests.
To help clinicians more easily gather and interpret the vast amount of patient data needed to make more personalized care recommendations, GE Healthcare is proud to introduce a series of new products and AI-powered solutions to the market and help expand access to precision health resources.
Nuclear Medicine (SPECT/CT)
Recently cleared by the FDA, GE Healthcare’s next-generation StarGuide SPECT/CT system uses the latest digital technologies to help clinicians improve patient outcomes in bone procedures, cardiology, neurology, oncology, and other medical specialties. The system’s cutting edge 12 CZT Digital Focus Detectors not only scan patients in 3D to provide more information to clinicians but they are also optimized for theranostic procedures.
The ability to generate high-quality SPECT/CT images starts with StarGuide’s unique Optical Scout technology, which leverages the system’s efficiency-focused Swift Plan workflow to determine the contour of the patient body and set the rest of the clinical scanning procedure into motion. After processing the Optical Scout data, StarGuide’s detectors and table automatically position themselves for close proximity and contactless scanning of the patient. The slim Digital Focus Detectors then orbit the body as closely as possible, and from all necessary angles, to scan the target area — and not the air surrounding the patient. The result is high-resolution images for clinicians and the minimization of time on the table for patients.
The excellent energy resolution of the GE Healthcare-produced CZT crystals for StarGuide’s Digital Focus Detectors offers clinicians the unique ability to simultaneously image multiple tracers in a single scan to help reduce the need for multiple patient visits and, in relevant cases, multiple patient sedations. Also, the combination of StarGuide’s shape adaptive gantry and CZT detector technology supports the imaging of tracers used in theranostics with impressive quality. This includes Lutetium-177 (177Lu), a tracer used to diagnose and evaluate a patient’s treatment response for neuroendocrine and prostate cancer.
GE Healthcare also recently announced Xeleris V, a new virtualized, flexible AI-powered solution that provides clinicians secure access to data from anywhere – helping them make personalized care decisions and treatment recommendations that are at the heart of precision health. This increase in access – paired with new AI-enabled applications and GE Healthcare’s large install base of nuclear medicine cameras – can simplify and enhance workflows to help clinicians quickly discover, diagnose, and treat patients with accuracy.
Market research shows that 73 percent of radiologists expect operational efficiency to be the main challenge in the next 1-3 years, while 64 percent of surveyed clinicians note that physician burnout has intensified during the pandemic. These statistics highlight a growing need for increased flexibility, access, and efficiency in healthcare today.
With these statistics in mind, Xeleris V’s new AI-enabled clinical applications work to streamline workflows, provide accurate data, and help expedite diagnoses across care areas: Q.Volumetrix AI, Q.Lung AI, and EXINI Bone.
PET/CT
GE Healthcare’s Discovery MI Gen 2 is the only PET/CT system that brings together the highest sensitivity of digital detection with the company’s industry-first CT image reconstruction technology: Deep Learning Image Reconstruction for TrueFidelity CT Images.
Generated using a dedicated deep neural network, TrueFidelity CT Images have the potential to improve reading confidence in a wide range of clinical applications such as head, whole body and cardiovascular, for patients of all ages. The system also offers Q.Clear for up to 2x improvement in image quality (SNR) as well as MotionFree for up to 67 percent improvement in lesion volume measurements.
Finally, Discovery MI Gen 2 is engineered for remote patient landmarking and positioning with AutoIN, which may aid in limiting contact with contagious diseases and unnecessary radiation exposure.
Radio Pharmacy
In December 2020, the FDA approved 68Gallium PSMA-11 (Ga 68 PSMA-11) – the first drug for PET imaging of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer, the third most common form of cancer in the United States., This approval is expected to increase demand for an already scarce PET imaging isotope: 68Gallium.
For many, a lack of access to radioisotopes can be a hinderance to practicing molecular imaging and precision health. Ongoing shortages of the generators that produce 68Gallium creates serious challenges for medical professionals treating a variety of patients.
GE Healthcare offers a solution with the expansion of its PETtrace cyclotron capabilities in combination with its FASTlab 2 Developer to now produce 68Gallium and increase theranostics capabilities.
Cyclotron produced 68Gallium is cheaper than generator production and provides a greater return on investment over the course of one year. This is largely due to a cyclotron’s ability to produce two times the amount of gallium chloride compared to a generator. In the last 15 years, 68Gallium publications increasing 100x and clinician interest and use of Gallium continues to grow.
Pharmaceutical Diagnostics & PET Tracers
In May 2021, GE Healthcare announced its acquisition of Zionexa, a leading innovator of in-vivo oncology and neurology biomarkers that help enable more personalized healthcare. With this acquisition, GE Healthcare aims to develop and bring to market Zionexa’s pipeline biomarkers, as well as the recently FDA-approved PET imaging agent, Cerianna (fluoroestradiol F-18), which is used as an adjunct to biopsy for the detection of estrogen receptor positive lesions to help inform treatment selection for patients with recurrent or metastatic breast cancer.
It is estimated that 168,000 people have metastatic breast cancer (“Stage 4”) in the U.S., with a five-year survival rate of 28 percent. Cerianna has been commercially available in the U.S. since December 2020 and today is accessible to approximately 25 percent of the relevant patient population. By leveraging its molecular imaging supply chain, R&D, medical affairs, market access, regulatory, quality and commercial expertise, GE Healthcare’s Pharmaceutical Diagnostics business aims to scale Cerianna to be accessible to a minimum of 75 percent of patients by 2023.
As the global leader in pharmaceutical imaging agents, GE Healthcare has a strong record of expanding clinical access to essential imaging agents, helping enable more personalized patient care. In 2021, the company shipped the 250,000th dose of DaTscan – which is an important neurology diagnostic used by clinicians to help inform treatment recommendations related to suspected Parkinsonian syndromes. Across all markets where it is available, DaTscan has been used to perform over one million scans to date.
While there is still much work to be done in the field of theranostics and precision health, the full breadth of GE Healthcare’s molecular imaging and pharmaceutical diagnostics portfolio offers clinicians unique opportunities to make personalized care decisions and treatment response assessments that are at the heart of personalized medicine and theranostics. GE Healthcare is the only partner with solutions spanning from pharmaceutical diagnostics, cyclotrons, chemistry synthesis, PET/CT, PET/MR, nuclear medicine, advanced digital solutions, and pharmaceutical partnerships to cover the breadth of steps from discovery to diagnosis to treatment.
For more information: gehealthcare.com


 May 30, 2024
May 30, 2024