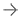Feinstein Institutes' Thomas Chaly, Ph.D., in front of a PET-CT imaging machine. He has been instrumental in pushing for FDA approval of a new PET imaging agent, Fluorodopa F-18 (FDOPA), to combat Parkinson’s
December 26, 2019 — The Feinstein Institutes for Medical Research has received U.S. Food and Drug Administration (FDA) approval for a new positron emission tomography (PET) drug to combat Parkinson’s disease. Fluorodopa F-18 (FDOPA) Injection (NDA 200655) will help visualize dopaminergic nerve terminals in the striatum for the evaluation of patients with suspected Parkinsonian syndromes.
Thomas Chaly, Ph.D., chief of cyclotron/radiochemistry at the Feinstein Institutes, was instrumental in getting this FDA approval. “This FDA approval is the pinnacle of our venture to have a safe and effective imaging agent for the differential diagnosis of Parkinsonian Syndromes,” said Chaly, who is also an associate investigator in the Institute of Molecular Medicine.
A PET scan is an imaging test that helps to understand the disease condition of the organs at the cellular level. PET uses a short-lived radiopharmaceutical to visually observe the affected area of the organ. In patients with Parkinson’s disease who experience loss of dopaminergic neurons, FDOPA uptake will be lower and this reduced uptake can be visually observed in the PET scan.
Fluorodopa F-18 Injection Helps Visualize Dopaminergic Nerve Terminals
Fluorodopa PET can be used to follow the disease’s progression. In addition, in patients with Parkinson’s disease who have received intracerebral transplantation of adrenal medulla tissue or fetal mesencephalic tissue, FDOPA PET may help to study the integrity and activity of the implant.
“With this approval, PET centers across the United States can incorporate FDOPA F-18 into their diagnostic and treatment follow up programs for patients with Parkinson’s disease,” Chaly said.
David Eidelberg, M.D., and Vijay Dhawan, Ph.D., helped direct the clinical studies for this NDA approval.
Fluorodopa F-18 Injection is injected into a vein in preparation for a PET scan to help detect the damaged or lost dopaminergic nerve cells. It is to be used in addition to other tests for diagnosing Parkinsonian syndromes.
To learn more about Fluorodopa F-18 trial.
Related Content:
PET Aids Dopamine Cell Transplantation for Parkinson’s Patients
Radiopharmaceutical Product Receives Positive Opinion in Europe


 May 17, 2024
May 17, 2024